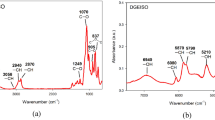
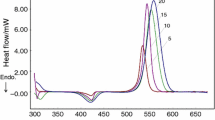
Abstract
Non-isothermal curing kinetics of DGEBA/PLA/MTHPA compounds was modeled using Ozawa, Kissinger, Friedman autocatalytic, Friedman and Málek models, whose parameters and associate deviation are reported. Ozawa and Kissinger consider global Ea over the whole conversion, estimated as 65.0 kJ/mol with R2 0.8393 and 87.60; these low R2 are due to the several stages during curing with distinct energy needs which most likely conducted to the discrepancies that should not be ignored; nevertheless, the average Ea might be adopted for the curing understanding, whereas adding PLA subtly increases Ea. Málek’s model adequately described the kinetics processes as also did isoconversional and autocatalytic Friedman models which presented R2 > 0.99. PLA’s molecular chains behaved as curing impediments, and during its progress, it is hypothesized that they increase the system’s swollen coils and eventually an interconnected structure that percolates the system results in (macro) gelation instead cross-linking; additionally, hydrogen bonds between PLA–DGEBA promote competitive reactions during reticulation.
Graphic abstract











Similar content being viewed by others
Data availability
Data will be made available on request.
References
M.R. Saeb et al., Biowaste chicken eggshell powder as a potential cure modifier for epoxy/anhydride systems: competitiveness with terpolymer-modified calcium carbonate at low loading levels. RSC Adv. 7, 2218–2230 (2017)
S. Kumar, S.K. Samal, S. Mohanty, S.K. Nayak, Curing kinetics of bio-based epoxy resin-toughened DGEBA epoxy resin blend. J. Therm. Anal. Calorim. 137, 1567–1578 (2019)
S. Kumar, S.K. Samal, S. Mohanty, S.K. Nayak, Recent development of biobased epoxy resins: a review. Polym.-Plast. Technol. Eng. 57, 133–155 (2018)
B. Ellis, Chemistry and Technology of Epoxy Resins (Springer, New York, 1993).
F.-L. Jin, X. Li, S.-J. Park, Synthesis and application of epoxy resins: a review. J. Ind. Eng. Chem. 29, 1–11 (2015)
V. Zvetkov, S. Djoumaliisky, E. Simeonova-Ivanova, The non-isothermal DSC kinetics of polyethylene tereftalate–epoxy compatible blends. Thermochim. Acta 553, 16–22 (2013)
X. Fernández-Francos, A. Rybak, R. Sekula, X. Ramis, A. Serra, Modification of epoxy–anhydride thermosets using a hyperbranched poly (ester-amide): I. Kinetic study. Polym. Int. 61, 1710–1725 (2012)
S. Montserrat, X. Pla, Use of temperature-modulated DSC in kinetic analysis of a catalysed epoxy–anhydride system. Polym. Int. 53, 326–331 (2004)
S. Montserrat, C. Flaque, M. Calafell, G. Andreu, J. Malek, Influence of the accelerator concentration on the curing reaction of an epoxy-anhydride system. Thermochim. Acta 269, 213–229 (1995)
S. Montserrat, J. Málek, A kinetic analysis of the curing reaction of an epoxy resin. Thermochim. Acta 228, 47–60 (1993)
Q. Xie et al., Structure, microparameters and properties of crosslinked DGEBA/MTHPA: a molecular dynamics simulation. AIP Adv. 8, 075332 (2018)
X. Yang et al., Molecular dynamics studies of the mechanical behaviors and thermal conductivity of the DGEBA/MTHPA/CNB composites. Composites B 164, 659–666 (2019)
Y. Cheng, S. Deng, P. Chen, R. Ruan, Polylactic acid (PLA) synthesis and modifications: a review. Front. Chem. China 4, 259–264 (2009)
X. Luo et al., A thermoplastic/thermoset blend exhibiting thermal mending and reversible adhesion. ACS Appl. Mater. Interfaces. 1, 612–620 (2009)
M. Abbate, E. Martuscelli, P. Musto, G. Ragosta, G. Scarinzi, Toughening of a highly cross-linked epoxy resin by reactive blending with bisphenol A polycarbonate. I. FTIR spectroscopy. J. Polym. Sci. Part B 32, 395–408 (1994)
K.A. Thakur, R.T. Kean, E.S. Hall, J.J. Kolstad, E.J. Munson, 1H NMR spectroscopy in the analysis and characterization of poly (lactide). Int. J. Polym. Anal. Charact. 4, 379–391 (1998)
I. Silva et al., Insights into the curing kinetics of epoxy/PLA: implications of the networking structure. Express Polym. Lett. 14, 1180–1196 (2020)
C. Acebo Gorostiza et al., Epoxy/anhydride thermosets modified with end-capped star polymers with poly (ethyleneimine) cores of different molecular weight and poly (epsilon-caprolactone) arms. Express Polym. Lett. 9, 809–823 (2015)
K.C. Cole, A new approach to modeling the cure kinetics of epoxy/amine thermosetting resins. 1. Mathematical development. Macromolecules 24, 3093–3097 (1991)
J. Mijovic, A. Fishbain, J. Wijaya, Mechanistic modeling of epoxy-amine kinetics. 1. Model compound study. Macromolecules 25, 979–985 (1992)
P. Šimon, Isoconversional methods. J. Therm. Anal. Calorim. 76, 123 (2004)
J. Criado, P. Sánchez-Jiménez, L. Pérez-Maqueda, Critical study of the isoconversional methods of kinetic analysis. J. Therm. Anal. Calorim. 92, 199–203 (2008)
Friedman, H.L. in Journal of Polymer Science Part C: Polymer Symposia. (Wiley Online Library), pp. 183–195.
M. Jouyandeh et al., Cure index’ for thermoset composites. Prog. Org. Coat. 127, 429–434 (2019)
E. Woo, J. Seferis, Cure kinetics of epoxy/anhydride thermosetting matrix systems. J. Appl. Polym. Sci. 40, 1237–1256 (1990)
D.W. Larsen, J.H. Strange, Pulsed NMR study of molecular motion in the uncured diglycidyl ether of bisphenol-A. J. Polym. Sci. Polym. Phys. Ed. 11, 65–74 (1973)
J. Zhang, L. Wang, S. Liu, Z. Li, Phosphazene/Lewis acids as highly efficient cooperative catalyst for synthesis of high-molecular-weight polyesters by ring-opening alternating copolymerization of epoxide and anhydride. J. Polym. Sci. 58, 803–810 (2020)
N.G. Jaques et al., Kinetic investigation of eggshell powders as biobased epoxy catalyzer. Compos. B 183, 107651 (2020)
S.H. Ryu, J. Sin, A. Shanmugharaj, Study on the effect of hexamethylene diamine functionalized graphene oxide on the curing kinetics of epoxy nanocomposites. Eur. Polym. J. 52, 88–97 (2014)
A. Ručigaj, B. Alič, M. Krajnc, U. Šebenik, Investigation of cure kinetics in a system with reactant evaporation: epoxidized soybean oil and maleic anhydride case study. Eur. Polym. J. 52, 105–116 (2014)
B. Wu et al., Super-toughened heat-resistant poly (lactic acid) alloys by tailoring the phase morphology and the crystallization behaviors. J. Polym. Sci. 58, 500–509 (2020)
H.E. Kissinger, Reaction kinetics in differential thermal analysis. Anal. Chem. 29, 1702–1706 (1957)
T. Ozawa, A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 38, 1881–1886 (1965)
J. Málek, The kinetic analysis of non-isothermal data. Thermochim. Acta 200, 257–269 (1992)
G.I. Senum, R. Yang, Rational approximations of the integral of the Arrhenius function. J. Therm. Anal. 11, 445–447 (1977)
K.A. Thakur, R.T. Kean, E.S. Hall, M.A. Doscotch, E.J. Munson, A quantitative method for determination of lactide composition in poly (lactide) using 1H NMR. Anal. Chem. 69, 4303–4309 (1997)
F.G. Garcia, B.G. Soares, Determination of the epoxide equivalent weight of epoxy resins based on diglycidyl ether of bisphenol A (DGEBA) by proton nuclear magnetic resonance. Polym. Test. 22, 51–56 (2003)
L. Li, Z. Zeng, H. Zou, M. Liang, Curing characteristics of an epoxy resin in the presence of functional graphite oxide with amine-rich surface. Thermochim. Acta 614, 76–84 (2015)
M. Nonahal et al., Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: nonisothermal cure kinetics study. Prog. Org. Coat. 114, 233–243 (2018)
Acknowledgments
The authors would like to thank Olin Corporation (Brazil) for kindly supplying the reactants. The authors are deeply grateful for the reading and discussions from Professor Tomas Jeferson de Mélo and Professor Pankaj Agrawal. The authors thank Prof. Dr. Marcelo Sobral da Silva, Prof. Dr. Josean Fechine Tavares, coordinators of the Multi-User Characterization and Analysis Laboratory—LMCA in UFPB, and Dr. Marcelo Felipe Rodrigues da Silva for the Nuclear Magnetic Resonance—NMR spectra.
Funding
The authors would like to acknowledge the financial support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Apoio à Pesquisa do Estado da Paraíba (FAPESQ) (Concession term: 017/2019). Professor Renate Wellen is CNPq fellow (Number: 307488/2018-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest and all authors have agreed with this submission and they are aware of the content.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, I.D.S., Barros, J.J.P., Jaques, N.G. et al. On the curing kinetics of epoxy/PLA compounds. Journal of Materials Research 36, 2973–2986 (2021). https://doi.org/10.1557/s43578-021-00234-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-021-00234-1