Abstract
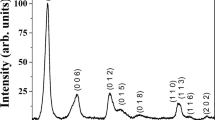
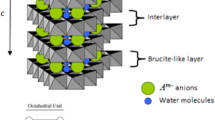
Aluminum lithium hydroxide carbonate hydrate, also known as Al-Li double hydroxide or Al-Li hydrotalcite-like compound [Al2Li(OH)6]2CO3·nH2O, was prepared from basic aluminum sulfate. This compound was prepared by precipitation in homogeneous solution of an aluminum bisulfite solution. A sodium aluminate aqueous solution was prepared by dissolving basic aluminum sulfate in 1M sodium hydroxide. The Al-Li double hydroxide was obtained after addition of lithium carbonate satured solution to the sodium aluminate solution, at 60 °C. The synthesized powder was characterized by thermal analysis (TG, DTG and DTA), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). By this method crystalline Li-Al hydrotalcite like compound with composition near to Al4Li2(OH)12CO3·3H2O was obtained.
Similar content being viewed by others
References
L. Ingram, HFW. Taylor, Mineral Mag. 36, 465–479 (1967).
A.V. Besserguenev, A. M. Fogg, R. J. Francis, S. J. Price, D. O’Hare, V. P. Isupov and B. P. Tolochko, Chem Mater 9, 241 (1997).
M. J. Climent, A. Corma, P. De Frutos, S. Iborra, M. Noy, A. Velty, P. Concepcion, Journal of Catalysis 269 (1), 140–149 (2010).
S. Miyata, M. Taketomi, T. Kojima, S. Tanizaki, A. Hashimoto, K. Kamishiro, M. Eguchi, S. Suzuki, Jpn. Patent No. 2000233188 (29 August 2000).
M. Del Arco, E. Cebadera, S. Gutierrez, C. Martin, MJ. Montero, V. Rives, J. Rocha, MA. Sevilla, J Pharm Sci 93, 1649–1658 (2004).
X. Duan et al., “Layered Double Hydroxides”, ed. X. Duan and D. G. Evans (Springer-Verlag Berlin Heidelberg, 2006) pp. 90–109.
R. A. Nyquist and R. O. Kagel, “Infrared Spectra of Inorganic Compounds”, (Academic Press, 1971) pp. 1–5.
K. Nakamoto, “Infrared and Raman Spectra of Inorganic and Coordination compounds”, (John Wiley & Sons, 1963) pp. 103–110.
R. L. Frost, M. L. Weier, and J. T. Kloprogge, Journal of Raman Spectroscopy 34 (10), 760–768 (2003).
J. T. Kloprogge, D. Wharton, L. Hickey and R. L. Frost, American Mineralogist 87 (5-6), 623–629 (2002).
C. J. Serna, J. L. Rendon and J. E. Iglesias, Clays and Clay Minerals 30 (3), 180–184 (1982).
C. A. Drewien, D. R. Tallant, M. O. Eatough, Journal of Materials Science 31 (16), 4321- 4325 (1996).
S. Miyata, Clays and Clay Minerals 28, 50–56 (1980).
Acknowledgments
The authors are grateful to Yolanda Gallaga O., V. Odemarys Vallejo T., and Juan Ignacio Macias for their contribution to the experimental work, and to Jeol Co. for SEM micrographs.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soto, C.A.C., Delgado, A.P., Ramírez, E.R. et al. Preparation and Characterization of Aluminum Lithium Hydroxide Carbonate Hydrate Obtained From Basic Aluminum Sulfate. MRS Online Proceedings Library 1372, 7–12 (2011). https://doi.org/10.1557/opl.2012.118
Published:
Issue Date:
DOI: https://doi.org/10.1557/opl.2012.118