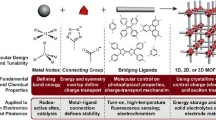
Abstract
Metal–organic frameworks (MOFs) are porous ordered arrays of inorganic clusters connected by organic linkers. The compositional diversity of the metal and ligand, combined with varied connectivity, has yielded more than 20,000 unique structures. Electronic structure theory can provide deep insights into the fundamental chemistry and physics of these hybrid compounds and identify avenues for the design of new multifunctional materials. In this article, a number of recent advances in materials modeling of MOFs are reviewed. We present the methodology for predicting the absolute band energies (ionization potentials) of porous solids as compared to those of standard semiconductors and electrical contacts. We discuss means of controlling the optical bandgaps by chemical modification of the organic and inorganic building blocks. Finally, we outline the principles for achieving electroactive MOFs and the key challenges to be addressed.




Similar content being viewed by others
References
K. Lejaeghere, T. Björkman, P. Blaha, S. Blügel, V. Blum, D. Caliste, I.E. Castelli, S.J. Clark, A. Dal Corso, S. de Gironcoli, T. Deutsch, J.K. Dewhurst, I. Di Marco, C. Draxl, M. Dułak, O. Eriksson, J.A. Flores-Livas, K.F. Garrity, L. Genovese, P. Giannozzi, M. Giantomassi, S. Goedecker, X. Gonze, O. Grånäs, E.K.U. Gross, A. Gulans, F. Gygi, D.R. Hamann, P.J. Hasnip, N.A.W. Holzwarth, D. Iuşan, D.B. Jochym, F. Jollet, D. Jones, G. Kresse, K. Koepernik, E. Küçükbenli, Y.O. Kvashnin, I.L.M. Locht, S. Lubeck, M. Marsman, N. Marzari, U. Nitzsche, L. Nordström, T. Ozaki, L. Paulatto, C.J. Pickard, W. Poelmans, M.I.J. Probert, K. Refson, M. Richter, G.-M. Rignanese, S. Saha, M. Scheffler, M. Schlipf, K. Schwarz, S. Sharma, F. Tavazza, P. Thunström, A. Tkatchenko, M. Torrent, D. Vanderbilt, M.J. van Setten, V. Van Speybroeck, J.M. Wills, J.R. Yates, G.-X. Zhang, S. Cottenier, Science 351, 1 (2016).
K.T. Butler, J.M. Frost, J.M. Skelton, K.L. Svane, A. Walsh, Chem. Soc. Rev., published online March 18, 2016, http://dx.doi.org/10.1039/C5CS00841G.
C.E. Wilmer, M. Leaf, C.Y. Lee, O.K. Farha, B.G. Hauser, J.T. Hupp, R.Q. Snurr, Nat. Chem. 4, 83 (2011).
http://gregchung.github.io/CoRE-MOFs.
Y.G. Chung, J. Camp, M. Haranczyk, B.J. Sikora, W. Bury, V. Krungleviciute, T. Yildirim, O.K. Farha, D.S. Sholl, R.Q. Snurr, Chem. Mater. 26, 6185 (2014).
M.T. Dove, Am. Mineral. 82, 213 (1997).
G. Kieslich, A.C. Forse, S. Sun, K.T. Butler, S. Kumagai, Y. Wu, M.R. Warren, A. Walsh, C.P. Grey, A.K. Cheetham, Chem. Mater. 28, 312 (2016).
G. Kieslich, S. Kumagai, K.T. Butler, T. Okamura, C.H. Hendon, S. Sun, M. Yamashita, A. Walsh, A.K. Cheetham, Chem. Commun. 51, 15538 (2015).
A.M.A. Leguy, J.M. Frost, A.P. McMahon, V. Garcia Sakai, W. Kockelmann, C.H. Law, X. Li, F. Foglia, A. Walsh, B.C. O’Regan, J. Nelson, J.T. Cabral, P.R.F. Barnes, Nat. Commun. 6, 7124 (2015).
F. Brivio, J.M. Frost, J.M. Skelton, A.J. Jackson, O.J. Weber, M.T. Weller, A.R. Goñi, A.M.A. Leguy, P.R.F. Barnes, A. Walsh, Phys. Rev. B Condens. Matter 92, 144308 (2015).
A.B. Cairns, A.L. Goodwin, Chem. Soc. Rev. 42, 4881 (2013).
J.P. Perdew, A.Ruzsinszky, G.I. Csonka, O.A. Vydrov, G.E. Scuseria, L.A. Constantin, X. Zhou, K. Burke, Phys. Rev. Lett. 100, 136406 (2008).
C.H. Hendon, D. Tiana, T.P. Vaid, A. Walsh, J. Mater. Chem. C 3, 95 (2013).
K. Svane, P.J. Saines, A. Walsh, J. Mater. Chem. C 3, 11076 (2015).
M. Nasalevich, C.H. Hendon, J.G. Santaclara, K. Svane, B. van der Linden, S.L. Veber, M.V. Fedin, A.J. Houtepen, M.A. van der Veen, F. Kapteijn, A. Walsh, J. Gascon, Sci. Rep. 6, 23676 (2016).
A.K. Cheetham, C.N.R. Rao, R.K. Feller, Chem. Commun. 46, 4780 (2006).
J.M. Frost, K.T. Butler, F. Brivio, C.H. Hendon, M. van Schilfgaarde, A. Walsh, Nano Lett. 14, 2584 (2014).
J.H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K.P. Lillerud, J. Am. Chem. Soc. 130, 13850 (2008).
H. Li, M. Eddaoudi, M. O’Keeffe, O.M. Yaghi, Nature 402, 276 (1999).
M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez, G. Férey, J. Am. Chem. Soc. 131, 10857 (2009).
L. Shen, R. Liang, M. Luo, F. Jing, L. Wu, Phys. Chem. Chem. Phys. 17, 117 (2015).
C.A. Allen, S.M. Cohen, Inorg. Chem. 53, 7014 (2014).
J.E. Mondloch, W. Bury, D. Fairen-Jimenez, S. Kwon, E.J. DeMarco, M.H. Weston, A.A. Sarjeant, S.T. Nguyen, P.C. Stair, R.Q. Snurr, O.K. Farha, J.T. Hupp, J. Am. Chem. Soc. 135, 10294 (2013).
D. Yang, S.O. Odoh, T.C. Wang, O.K. Farha, J.T. Hupp, C.J. Cramer, L. Gagliardi, B.C. Gates, J. Am. Chem. Soc. 137, 7391 (2015).
H. Fei, S.M. Cohen, J. Am. Chem. Soc. 137, 2191 (2015).
C.K. Brozek, M. Dincă, J. Am. Chem. Soc. 135, 12886 (2013).
C.H. Hendon, D. Tiana, M. Fontecave, C. Sanchez, L. D’arras, C. Sassoye, L. Rozes, C. Mellot-Draznieks, A. Walsh, J. Am. Chem. Soc. 135, 10942 (2013).
K.T. Butler, C.H. Hendon, A. Walsh, J. Am. Chem. Soc. 136, 2703 (2014).
H. Kroemer, Nobel Lecture, “Quasi-Electric Fields and Band Offsets: Teaching Electrons New Tricks” (2000).
J. Bardeen, Phys. Rev. 49, 635 (1936).
A. Walsh, K.T. Butler, Acc. Chem. Res. 47, 364 (2014).
E. Smith, Physica A 120A, 327 (1983).
J. Ihm, A. Zunger, M. Cohen, J. Phys. C Solid State Phys. 12, 4409 (1979).
J. Buckeridge, K.T. Butler, C.R.A. Catlow, A.J. Logsdail, D.O. Scanlon, S.A. Shevlin, S.M. Woodley, A.A. Sokol, A. Walsh, Chem. Mater. 27, 3844 (2015).
D.O. Scanlon, C.W. Dunnill, J. Buckeridge, S.A. Shevlin, A.J. Logsdail, S.M. Woodley, C.R.A. Catlow, M.J. Powell, R.G. Palgrave, I.P. Parkin, G.W. Watson, T.W. Keal, P. Sherwood, A. Walsh, A.A. Sokol, Nat. Mater. 12, 798 (2013).
https://github.com/WMD-group/MacroDensity.
Z.-L. Wu, C.-H. Wang, B. Zhao, J. Dong, F. Lu, W.-H. Wang, W.-C. Wang, G.-J. Wu, J.-Z. Cui, P. Cheng. Angew. Chem. Int. Ed. Engl. 55, 4938 (2016).
S. Hamad, N.C. Hernandez, A. Aziz, A.R. Ruiz-Salvador, S. Calero, R. Grau-Crespo, J. Mater. Chem. A 3, 23458 (2015).
K.T. Butler, Y. Kumagai, F. Oba, A. Walsh, J. Mater. Chem. C 4, 1149 (2016).
G.A. Ozin, Adv. Mater. 4, 612 (1992).
C.H. Hendon, A. Walsh, Chem. Sci. 6, 3674 (2015).
C.H. Hendon, D. Tiana, A.T. Murray, D.R. Carbery, A. Walsh, Chem. Sci. 4, 4278 (2013).
D. Tiana, C. Hendon, A. Walsh, Chem. Commun. 50, 13990 (2014).
A.A. Talin, A. Centrone, A.C. Ford, M.E. Foster, V. Stavila, P. Haney, R.A. Kinney, V. Szalai, F. El Gabaly, H.P. Yoon, F. Léonard, M.D. Allendorf, Science 343, 6166 (2014).
J.K. Bristow, K.L. Svane, D. Tiana, J.M. Skelton, J.D. Gale, A. Walsh, J. Phys. Chem. C 120, 9276 (2016).
K.T. Butler, C.H. Hendon, A. Walsh, ACS Appl. Mater. Interfaces 6, 22044 (2014).
C.H. Hendon, K.E. Wittering, T.-H. Chen, W. Kaveevivitchai, I. Popov, K.T. Butler, C.C. Wilson, D.L. Cruickshank, O.Š. Miljanić, A. Walsh, Nano Lett. 15, 2149 (2015).
Web of Science, June 2016.
M.D. Allendorf, A. Schwartzberg, V. Stavila, A.A. Talin, Chem. Eur. J. 17, 11372 (2011).
Acknowledgments
We thank W. Kohn and H. Kroto for the development of density functional theory and physical properties of MOF chemistry, respectively, as well as for stimulating lectures and discussions on these topics. The research discussed here has benefited from collaboration with J.K. Bristow, D. Tiana, and K.L. Svane. We acknowledge support from The Royal Society, the European Research Council (Grant No. 27757) and the EPSRC (Grant No. EP/M009580/1 and EP/K016288/1). This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation Grant Number ACI-1053575.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walsh, A., Butler, K.T. & Hendon, C.H. Chemical principles for electroactive metal–organic frameworks. MRS Bulletin 41, 870–876 (2016). https://doi.org/10.1557/mrs.2016.243
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrs.2016.243