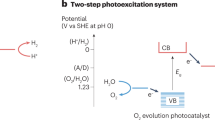
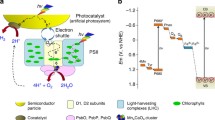
Abstract
Water splitting to produce H2 using sunlight is a form of artificial photosynthesis in that light energy is converted to chemical energy. As such, water splitting using powdered photocatalysts has attracted attention in the framework of energy and environmental issues. This article reviews z-scheme photocatalyst systems for water splitting under visible light irradiation, especially focused on the systems consisting of SrTiO3:Rh of a H2-evolving photocatalyst, and O2-evolving photocatalysts with and without electron mediators. These photocatalyst systems showed activities for water splitting into H2 and O2 in a stoichiometric amount under visible light irradiation and even under sunlight irradiation. The photocatalytic activity was sensitive to pH. The optimum pH was 2.4 when iron ions were used as electron mediators. Co-catalysts also affected the activity. The photodeposited Ru co-catalyst gave an excellent performance. The best performance achieved by the pH adjustment and the selection of a co-catalyst was obtained mainly by suppression of back reactions to form H2O from evolved H2 and O2.









Similar content being viewed by others
References
A. Fujishima, K. Honda, Nature 238, 37 (1972).
O. Khaselev, J.A. Turner, Science 280, 425 (1998).
M. Grätzel, Nature 414, 338 (2001).
S. Licht, J. Phys. Chem. B 105, 6281 (2001).
A. Kudo, Int. J. Hydrogen Energy 32, 2673 (2007).
K. Domen, M. Hara, J.N. Kondo, T. Takata, Bull. Chem. Soc. Jpn. 73, 1307 (2000).
H. Arakawa, K. Sayama, Catal. Surveys Jpn. 4, 75 (2000).
K. Maeda, K. Domen, J. Phys. Chem. C 111, 7851 (2007).
F.E. Osterloh, Chem. Mater. 20, 35 (2008).
A. Kudo, Y. Miseki, Chem. Soc. Rev. 38, 253 (2009).
Y. Inoue, Energy Environ. Sci. 2, 364 (2009).
K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue, K. Domen, Nature 440, 295 (2006).
Y. Lee, H. Terashita, Y. Shimodaira, K. Teramura, M. Hara, H. Kobayashi, K. Domen, M. Yashima, J. Phys. Chem. C 111, 1042 (2007).
J.R. Darwent, A. Mills, J. Chem. Soc., Faraday Trans. 2 78, 359 (1982).
A. Kudo, K. Omori, H. Kato, J. Am. Chem. Soc. 121, 11459 (1999).
H. Kato, A. Kudo, J. Phys. Chem. B106, 5029 (2002).
H. Kato, H. Kobayashi, A. Kudo, J. Phys. Chem. B 106, 12441 (2002).
T. Ishii, H. Kato, A. Kudo, J. Photochem. Photobiol. A 163, 181 (2004)
R. Konta,T. Ishii, H. Kato, A. Kudo, J. Phys. Chem. B 108, 8992 (2004).
Y. Hosogi, K. Tanabe, H. Kato, H. Kobayashi, A. Kudo, Chem. Lett. 33, 28 (2004).
Y. Shimodaira, H. Kato, H. Kobayashi, A. Kudo, Bull. Chem. Soc. Jpn. 80, 885 (2007).
R. Niishiro, R. Konta, H. Kato, W.J. Chun, K. Asakura, A. Kudo, J. Phys. Chem. C 111, 17420 (2007).
A. Ishikawa, T. Takata, J.N. Kondo, M. Hara, H. Kobayashi, K. Domen, J. Am. Chem. Soc. 124, 13547 (2002).
D. Yamashita, T. Takata, M. Hara, J.N. Kondo, K. Domen, Solid State Ionics. 172, 591 (2004).
K. Sayama, K. Mukasa, R. Abe, Y Abe, H. Arakawa, Chem. Commun. 2416 (2001).
H. Kato, M. Hori, R. Konta, Y Shimodaira, A. Kudo, Chem. Lett. 33, 1348 (2004).
R. Abe, T. Takata, H. Sugihara, K. Domen, Chem. Commun. 3829 (2005).
H. Kato, Y Sasaki, A. Iwase, A. Kudo, Bull. Chem. Soc. Jpn. 12, 2457 (2007).
M. Higashi, R. Abe, A. Ishikawa, T. Takata, B. Ohtani, K. Domen, Chem. Lett. 37, 138 (2008).
M. Higashi, R. Abe, K. Teramura, T. Takata, B. Ohtani, K. Domen, Chem. Phys. Lett. 452,120 (2008).
Y. Sasaki, A. Iwase, H. Kato, A. Kudo, J. Catal. 259,133 (2008).
R. Abe, K. Shinmei, K. Hara, B. Ohtani, Chem. Commun. 3577 (2009).
K. Maeda, M. Higashi, D. Lu, R. Abe, K. Domen, J. Am. Chem. Soc. 132, 5858 (2010).
S. Ogura, M. Kohno, K. Saito, Y. Inoue, Phys. Chem. Chem. Phys. 1, 179 (1999).
Y. Lee, H. Terashima, Y. Shimodaira, K. Teramura, M. Hara, H. Kobayashi, K. Domen, M. Yashima, J. Phys. Chem. C111, 1042 (2007).
T. Sakata, K. Hashimoto, T. Kawai, J. Phys. Chem. 88, 5214 (1984).
Y. Sasaki, H. Nemoto, K. Saito, A. Kudo, J. Phys. Chem. C 113, 17536 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudo, A. Z-scheme photocatalyst systems for water splitting under visible light irradiation. MRS Bulletin 36, 32–38 (2011). https://doi.org/10.1557/mrs.2010.3
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrs.2010.3