Abstract
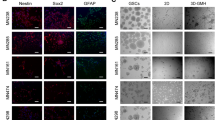
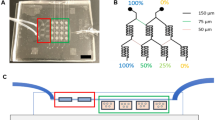
While preclinical models such as orthotopic tumors generated in mice from patient-derived specimens are widely used to predict sensitivity or therapeutic interventions for cancer, such xenografts can be slow, require extensive infrastructure, and can make in situ assessment difficult. Such concerns are heightened in highly aggressive cancers, such as glioblastoma (GBM), that display genetic diversity and short mean survival. Biomimetic biomaterial technologies offer an approach to create ex vivo models that reflect biophysical features of the tumor microenvironment (TME). We describe a microfluidic templating approach to generate spatially graded hydrogels containing patient-derived GBM cells to explore drug efficacy and resistance mechanisms.




Similar content being viewed by others
References
D.N. Louis, A. Perry, G. Reifenberger, A. von Deimling, D. Figarella-Branger, W.K. Cavenee, H. Ohgaki, O.D. Wiestler, P. Kleihues, and D.W. Ellison: The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803 (2016).
F.B. Furnari, T. Fenton, R.M. Bachoo, A. Mukasa, J.M. Stommel, A. Stegh, W.C. Hahn, K.L. Ligon, D.N. Louis, C. Brennan, L. Chin, R.A. DePinho, and W.K. Cavenee: Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 21, 2683 (2007).
D.R. Johnson and B.P. O’Neill: Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 107, 359 (2012).
N.A. Charles, E.C. Holland, R. Gilbertson, R. Glass, and H. Kettenmann: The brain tumor microenvironment. Glia 59, 1169 (2011).
C. Jackson, J. Ruzevick, J. Phallen, Z. Belcaid, and M. Lim: Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin. Dev. Immunol. 2011, 20 (2011).
R. Stupp, W.P. Mason, M.J. van den Bent, M. Weller, B. Fisher, M.J. Taphoorn, K. Belanger, A.A. Brandes, C. Marosi, U. Bogdahn, J. Curschmann, R.C. Janzer, S.K. Ludwin, T. Gorlia, A. Allgeier, D. Lacombe, J.G. Cairncross, E. Eisenhauer, and R.O. Mirimanoff: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987 (2005).
M. Okada, M. Saio, Y. Kito, N. Ohe, H. Yano, S. Yoshimura, T. Iwama, and T. Takami: Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int. J. Oncol. 34, 1621 (2009).
J.D. Lathia, S.C. Mack, E.E. Mulkearns-Hubert, C.L. Valentim, and J.N. Rich: Cancer stem cells in glioblastoma. Genes Dev. 29, 1203 (2015).
N.G. Thaker and I.F. Pollack: Molecularly targeted therapies for malignant glioma: rationale for combinatorial strategies. Expert Rev. Neurother. 9, 1815 (2009).
T.T. Huang, S.M. Sarkaria, T.F. Cloughesy, and P.S. Mischel: Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics 6, 500 (2009).
S. Misra, B.P. Toole, and S. Ghatak: Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J. Biol. Chem. 281, 34936 (2006).
A. Rape, B. Ananthanarayanan, and S. Kumar: Engineering strategies to mimic the glioblastoma microenvironment. Adv. Drug Delivery. Rev. 79–80, 172 (2014).
P. Roth and M. Weller: Challenges to targeting epidermal growth factor receptor in glioblastoma: escape mechanisms and combinatorial treatment strategies. Neuro Oncol. 16, viii14 (2014).
T.E. Taylor, F.B. Furnari, and W.K. Cavenee: Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr. Cancer Drug Targets 12, 197 (2012).
A. Schulte, K. Liffers, A. Kathagen, S. Riethdorf, S. Zapf, A. Merlo, K. Kolbe, M. Westphal, and K. Lamszus: Erlotinib resistance in EGFR-amplified glioblastoma cells is associated with upregulation of EGFRvIII and PI3Kp110δ. Neuro Oncol. 15, 1289 (2013).
M.G. Slomiany, L. Dai, P.A. Bomar, T.J. Knackstedt, D.A. Kranc, L. Tolliver, B.L. Maria, and B.P. Toole: Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res. 69, 4992 (2009).
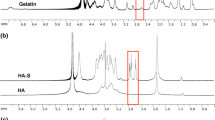
S. Pedron, E. Becka, and B.A. Harley: Spatially gradated hydrogel platform as a 3D engineered tumor microenvironment. Adv. Mater. 27, 1567 (2015).
J.M. Heddleston, M. Hitomi, M. Venere, W.A. Flavahan, K. Yan, Y. Kim, S. Minhas, J.N. Rich, and A.B. Hjelmeland: Glioma stem cell maintenance: the role of the microenvironment. Curr. Pharm. Des. 17, 2386 (2011).
R.G.W. Verhaak, K.A. Hoadley, E. Purdom, V. Wang, Y. Qi, M.D. Wilkerson, C.R. Miller, L. Ding, T. Golub, J.P. Mesirov, G. Alexe, M. Lawrence, M. O’Kelly, P. Tamayo, B.A. Weir, S. Gabriel, W. Winckler, S. Gupta, L. Jakkula, H.S. Feiler, J.G. Hodgson, C.D. James, J.N. Sarkaria, C. Brennan, A. Kahn, P.T. Spellman, R.K. Wilson, T.P. Speed, J.W. Gray, M. Meyerson, G. Getz, C.M. Perou, D.N. Hayes, and N. Canc Genome Atlas Res: Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98 (2010).
B. Westermark: Glioblastoma—a moving target. Ups. J. Med. Sci. 117, 251 (2012).
D. Hambardzumyan, Y.-K. Cheng, H. Haeno, E.C. Holland, and F. Michor: The probable cell of origin of NF1- and PDGF-driven glioblastomas. PLoS ONE 6, e24454 (2011).
M. Labussiere, M. Sanson, A. Idbaih, and J.Y. Delattre: IDH1 gene mutations: a new paradigm in glioma prognosis and therapy? Oncologist 15, 196 (2010).
J.N. Rich, C. Hans, B. Jones, E.S. Iversen, R.E. McLendon, B.K. Rasheed, A. Dobra, H.K. Dressman, D.D. Bigner, J.R. Nevins, and M. West: Gene expression profiling and genetic markers in glioblastoma survival. Cancer Res. 65, 4051 (2005).
J.N. Sarkaria, L. Yang, P.T. Grogan, G.J. Kitange, B.L. Carlson, M.A. Schroeder, E. Galanis, C. Giannini, W. Wu, E.B. Dinca, and C.D. James: Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol. Cancer Ther. 6, 1167 (2007).
J.N. Sarkaria, B.L. Carlson, M.A. Schroeder, P. Grogan, P.D. Brown, C. Giannini, K.V. Ballman, G.J. Kitange, A. Guha, A. Pandita, and C.D. James: Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin. Cancer Res. 12, 2264 (2006).
C. Giannini, J.N. Sarkaria, A. Saito, J.H. Uhm, E. Galanis, B.L. Carlson, M.A. Schroeder, and C.D. James: Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 7, 164 (2005).
S. Pedron, E. Becka, and B.A.C. Harley: Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials 34, 7408 (2013).
B.P. Mahadik, T.D. Wheeler, L.J. Skertich, P.J. Kenis, and B.A. Harley: Microfluidic generation of gradient hydrogels to modulate hematopoietic stem cell culture environment. Adv. Healthc. Mater. 3, 449 (2014).
B.P. Mahadik, S. Pedron Haba, L.J. Skertich, and B.A.C. Harley: The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials 67, 297 (2015).
T. Mosmann: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55 (1983).
G.P. Duffy, T.M. McFadden, E.M. Byrne, S.L. Gill, E. Farrell, and F.J. O’Brien: Towards in vitro vascularisation of collagen-GAG scaffolds. Eur. Cells Mater. 21, 15 (2011).
K.J. Livak and T.D. Schmittgen: Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402 (2001).
M.R. Wiranowska and M. V. Rojiani: Extracellular matrix microenvironment in glioma progression, in Glioma—Exploring Its Biology and Practical Relevance, edited by A. Ghosh (InTech, Rijeka, Croatia, 2011), p. 257.
R. Endersby and S.J. Baker: PTEN signaling in brain: neuropathology and tumorigenesis. Oncogene 27, 5416 (2008).
A. Perez, D.M. Neskey, J. Wen, L. Pereira, E.P. Reategui, W.J. Goodwin, K.L. Carraway, and E.J. Franzmann: CD44 interacts with EGFR and promotes head and neck squamous cell carcinoma initiation and progression. Oral Oncol. 49, 306 (2013).
J. Cha, S.-G. Kang, and P. Kim: Strategies of mesenchymal invasion of patient-derived brain tumors: microenvironmental adaptation. Sci. Rep. 6, 24912 (2016).
B.P. Toole: Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 4, 528 (2004).
D. Tsatas, V. Kanagasundaram, A. Kaye, and U. Novak: EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J. Clin. Neurosci. 9, 282 (2002).
J.-W. Chen, S. Pedron and B.A.C. Harley: The combined influence of hydrogel stiffness and matrix-bound hyaluronic acid content on glioblastoma invasion. Macromol. Biosci. 17, 1616 (2017).
R.L. Klank, S.A. Decker Grunke, B.L. Bangasser, C.L. Forster, M.A. Price, T.J. Odde, K.S. SantaCruz, S.S. Rosenfeld, P. Canoll, E.A. Turley, J.B. McCarthy, J.R. Ohlfest, and D.J. Odde: Biphasic dependence of glioma survival and cell migration on CD44 expression level. Cell Rep. 18, 23.
J. Mendelsohn and J. Baselga: Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 21, 2787 (2003).
R.W. Akita and M.X. Sliwkowski: Preclinical studies with erlotinib (Tarceva). Semin. Oncol. 30, 15 (2003).
S.J. Wang and L.Y.W. Bourguignon: Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 178, 956 (2011).
C. Holohan, S. Van Schaeybroeck, D.B. Longley, and P.G. Johnston: Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714 (2013).
R. Ohashi, F. Takahashi, R. Cui, M. Yoshioka, T. Gu, S. Sasaki, S. Tominaga, K. Nishio, K.K. Tanabe, and K. Takahashi: Interaction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cell. Cancer Lett. 252, 225 (2007).
Acknowledgments
The authors are grateful for seed funding provided by the Illini 4000 as well as the by Mayo Clinic—University of Illinois Alliance for Technology-Based Healthcare. Research reported in this publication was also supported by the National Cancer Institute of the National Institutes of Health under Award Number R01 CA197488. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors are also grateful for additional funding provided by the Illinoi4000, the Department of Chemical and Biomolecular Engineering, and the Carl R. Woese Institute for Genomic Biology at the University of Illinois at Urbana-Champaign.
Author information
Authors and Affiliations
Corresponding author
Supplementary material
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1557/mrc.2017.85.
Rights and permissions
About this article
Cite this article
Pedron, S., Polishetty, H., Pritchard, A.M. et al. Spatially graded hydrogels for preclinical testing of glioblastoma anticancer therapeutics. MRS Communications 7, 442–449 (2017). https://doi.org/10.1557/mrc.2017.85
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2017.85