Abstract
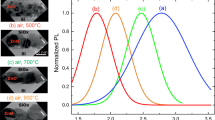
We report the synthesis and optical properties of pure ZnS and Ag doped ZnS nanostructures. ZnS(Ag) was synthesized by using the hydrothermal technique and later annealed at different temperatures under vacuum conditions. It was observed that the photoluminescence (PL) emission from the ZnS(Ag) nanostructures can be easily tuned from the blue (445 nm) to green (530 nm) region of visible light by varying the annealing temperature. This tunability has been attributed to the introduction of excess sulfur vacancy states, which is evident from the PL excitation spectra. This observed change in the PL emission wavelength can be highly beneficial in the imaging screens where ZnS is regularly used and can be easily interfaced with the silicon photodiodes showing maximum sensitivity at 550 nm.







Similar content being viewed by others
References
P. Sunghoon, A. Soyeon, K. Hyunsung, S. Lee, and C. Lee: Synthesis, structure and UV-enhanced gas sensing properties of Au-functionalized ZnS nano-wires. Sens. Actuators, B 188, 1270–1276 (2013).
A.K. Kole, C.S. Tiwary, and P. Kumbhakar: Morphology controlled synthesis of wurtzite ZnS nanostructures through simple hydrothermal method and observation of white light emission from ZnO obtained by annealing the synthesized ZnS nanostructures. J. Mater. Chem. C 2, 4338 (2014).
S.S. Boxi and S. Paria: Effect of silver doping on TiO2, CdS, and ZnS nanoparticles for the photocatalytic degradation of metronidazole under visible light. RSC Adv. 4, 37752 (2014).
Y. Liang, H. Liang, X. Xiaoa, and S. Hark: The epitaxial growth of ZnS nanowire arrays and their applications in UV-light detection. J. Mater. Chem. 22, 1199–1205 (2012).
J.S. McCloy, M. Bliss, B. Miller, Z. Wang, and S. Stave: Scintillation and luminescence in transparent colorless single and polycrystalline bulk ceramic ZnS. J. Lumin. 157, 416–423 (2015).
X. Fang, T. Zhai, U.K. Gautam, L. Li, L. Wu, Y. Bando, and D. Golberg: ZnS nanostructures: From synthesis to applications. Prog. Mater. Sci. 56, 175–287 (2011).
Z. Li, B. Liu, X. Li, S. Yu, L. Wang, Y. Hou, Y. Zou, M. Yao, Q. Li, B. Zou, G. Zou, G. Wang, and Y. Liu: Synthesis of ZnS nanocrystals with controllable structure and morphology and their photoluminescence property. Nanotechnol. 18, 255602 (2007).
R. Mendil, Z.B. Ayadi, and K. Djessas: Effect of solvent medium on the structural, morphological and optical properties of ZnS nanoparticles synthesized by solvothermal route. J. Alloys Compd. 678, 87–92 (2016).
B.Y. Geng, X.W. Liu, Q.B. Du, X.W. We, and L.D. Zhang: Structure and optical properties of periodically twinned ZnS nanowires. Appl. Phys. Lett. 88, 163104 (2006).
N. Murase, R. Jagannathan, Y. Kanematsu, M. Watanbe, A. Kurita, K. Hirata, T. Yazawa, and T. Kushida: Fluorescence and EPR characteristics of Mn2+-doped ZnS nanocrystals prepared by aqueous colloidal method. J. Phys. Chem. B 103, 754 (1999).
K.B. Lin and Y.H. Su: Photoluminescence of Cu:ZnS, Ag:ZnS, and Au:ZnS nanoparticles applied in Bio-LED. Appl. Phys. B 113, 351–359 (2013).
L. Lu, W. Zeng, S. Hu, D. Chen, J. Lei, and N. Ren: Polarization-dependent fluorescence of CdSe/ZnS quantum dots coupling to a single gold-silver alloy nanotube. J. Alloys Compd. 731, 753–759 (2017).
X.J. Xu, L.F. Hu, N. Gao, S. Liu, S. Wageh, A.A. Al-Ghamdi, A. Alshahrie, and X. Fang: Controlled growth from ZnS nanoparticles to ZnS–CdS nanoparticle hybrids with enhanced photo- activity. Adv. Funct. Mater. 25, 445–454 (2015).
E. Hao, Y. Sun, B. Yang, X. Zhang, J. Liu, and J. Shen: Synthesis and photophysical properties of ZnS colloidal particles doped with silver. J. Colloid Interface Sci. 204, 369–373 (1998).
Q. Pan, D. Yang, Y. Zhao, Z. Ma, G. Dong, and J. Qiu: Facile hydrothermal synthesis of Mn doped ZnS nanocrystals and luminescence properties investigations. J. Alloys Compd. 579, 300–304 (2013).
J-C. Lee and D-H. Park: Self-defects properties of ZnS with sintering temperature. Mater. Lett. 57, 2872–2878 (2003).
W. Zhang, X. Zeng, H. Liu, and J. Lu: Synthesis and investigation of blue and green emissions of ZnS ceramics. J. Lumin. 134, 498–503 (2013).
P. Yang, M. Lu, D. Xu, D.L. Yuan, and G.J. Zhou: Photoluminescence properties of ZnS nanoparticles co-doped with Pb2+ and Cu2+. Chem. Phys. Lett. 336, 76–80 (2001).
C. Ye, X. Fang, G. Li, and L. Zhang: Origin of the green photoluminescence from zinc sulfide nanobelts. Appl. Phys. Lett. 85, 3035–3037 (2004).
S.A. Acharya, N. Maheshwari, L. Tatikondewar, A. Kshirsagar, and S.K. Kulkarni: Ethylenediamine-mediated wurtzite phase formation in ZnS. Cryst. Growth Des. 13, 1369–1376 (2013).
M. Fantauzzi, B. Elsener, D. Atzei, A. Rigoldiab, and A. Rossiab: Exploiting XPS for the identification of sulfides and polysulfides. RSC Adv. 5, 75953–75963 (2015).
G. Hota, S.B. Idage, and K.C. Khilar: Characterization of nano-sized CdS–Ag2S core-shell nanoparticles using XPS technique. Colloids Surf., A 293, 5–12 (2007).
S. Pan, X. Liu, and X. Wang: Preparation of Ag2S–Graphene nanocomposite from a single source precursor and its surface-enhanced Raman scattering and photoluminescent activity. Mater. Charact. 62, 1094–1101 (2011).
W. Becker and A.J. Bard: Photoluminescence and photoinduced oxygen adsorption of colloidal Zinc Sulfide dispersions. J. Phys. Chem. 87, 4888–4893 (1983).
D. Denzler, M. Olschewski, and K. Sattler: Luminescence studies of localized gap states in colloidal ZnS nanocrystals. J. Appl. Phys. 84, 2841–2845 (1998).
W.Q. Peng, G.W. Cong, S.C. Qu, and Z.G. Wang: Synthesis and photoluminescence of ZnS:Cu nanoparticles. Opt. Mater. 29, 313–317 (2006).
Q. Xiong, G. Chen, J.D. Acord, X. Liu, J.J. Zengel, H.R. Gutierrez, J.M. Redwing, L.C. Lew Yan Voon, B. Lassen, and P.C. Eklund: Optical properties of rectangular cross-sectional ZnS nanowires. Nano Lett. 4, 1663–1668 (2004).
S. Kakarndee, S. Juabrum, and S. Nanan: Low temperature synthesis, characterization and photoluminescence study of plate-like ZnS. Mater. Lett. 164, 198–201 (2016).
ACKNOWLEDGMENTS
The authors would like to thank GAMD, BARC for SEM measurements. M. Sharma would like to thank DST for INSPIRE Faculty grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, M., Sen, S., Gupta, J. et al. Tunable blue-green emission from ZnS(Ag) nanostructures grown by hydrothermal synthesis. Journal of Materials Research 33, 3963–3970 (2018). https://doi.org/10.1557/jmr.2018.358
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2018.358