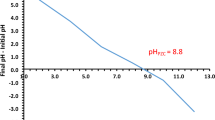
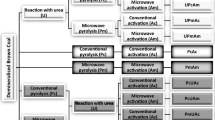
Abstract
Manufacturing of advanced functional materials should also rely on the green chemistry principles like utilization of natural renewable resources. Marine environment offers plenty of renewable raw materials like chitin and its derivative chitosan. The paper presents how urea treatment has influenced several textural, chemical, and electrocatalytic properties of N-doped activated carbons (N_ACs) obtained from chitosan and chitin. The materials were subjected to an activation procedure (with different activators) as well as nitrogenation by premixing the precursors with water solutions of urea. Raw and premixed precursors were carbonized in the temperature range of 700–800 °C. The urea treatment resulted in a spectacular increase in the nitrogen content by weight (up to 68%) and an improvement of the surface area (up to 42%) along with total/micro-/mezo-pore volume (up to 49%). Some urea-modified N_ACs were capable of reducing oxygen in an alkaline solution as effectively as a Pt-loaded carbon material. The highest number of electrons transferred to O2 molecule was found to be equal to 3.76 for a chitosan derived sample. This ability of chitosan and chitin derived N-rich activated carbons was studied by means of the method named rotating ring disc electrode.







Similar content being viewed by others
References
D. Hulicova-Jurcakova, M. Seredych, G.Q. Lu, N. Kodiweera, P.E. Stallworth, S. Greenbaum, and T.J. Bandosz: Effect of surface phosphorus functionalities of activated carbons containing oxygen and nitrogen on electrochemical capacitance. Carbon 47, 1576 (2009).
C. Wang, L. Sun, Y. Zhou, P. Wan, X. Zhang, and J. Qiu: P/N co-doped microporous carbons from H3PO4-doped polyaniline by in situ activation for supercapacitors. Carbon 59, 537 (2013).
Y.J. Kim, Y. Abe, T. Yanagiura, K.C. Park, M. Shimizu, T. Iwazaki, S. Nakagawa, M. Endo, and M.S. Dresselhaus: Easy preparation of nitrogen-enriched carbon materials from peptides of silk fibroins and their use to produce a high volumetric energy density in supercapacitors. Carbon 45, 2116 (2007).
K. László, E. Tombácz, and K. Josepovits: Effect of activation on the surface chemistry of carbons from polymer precursors. Carbon 39, 1217 (2001).
L. Li, E. Liu, J. Li, Y. Yang, H. Shen, Z. Huang, X. Xiang, and W. Li: A doped activated carbon prepared from polyaniline for high performance supercapacitors. J. Power Sources 195, 1516 (2010).
Y. Deng, Y. Xie, K. Zou, and X. Ji: Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 4, 1144 (2016).
M. Kodama, J. Yamashita, Y. Soneda, H. Hatori, and K. Kamegawa: Preparation and electrochemical characteristics of N-enriched carbon foam. Carbon 45, 1105 (2007).
J. Klinik, B. Samojeden, T. Grzybek, W. Suprun, H. Papp, and R. Gläser: Nitrogen promoted activated carbons as DeNOx catalysts. 2. The influence of water on the catalytic performance. Catal. Today 176, 303 (2011).
E. Fiset, T.E. Rufford, M. Seredych, T.J. Bandosz, and D. Hulicova-Jurcakova: Comparison of melamine resin and melamine network as precursors for carbon electrodes. Carbon 81, 239 (2015).
C. Qin, X. Lu, G. Yin, Z. Jin, Q. Tan, and X. Bai: Study of activated nitrogen-enriched carbon and nitrogen-enriched carbon/carbon aerogel composite as cathode materials for supercapacitors. Mater. Chem. Phys. 126, 453 (2011).
B. Xu, S. Hou, G. Cao, F. Wu, and Y. Yang: Sustainable nitrogen-doped porous carbon with high surface areas prepared from gelatin for supercapacitors. J. Mater. Chem. 22, 19088 (2012).
B. Zhang, Z. Wen, S. Ci, S. Mao, J. Chen, and Z. He: Synthesizing nitrogen-doped activated carbon and probing its active sites for oxygen reduction reaction in microbial fuel cells. ACS Appl. Mater. Interfaces 6, 7464 (2014).
K. Jurewicz, K. Babeł, A. Ziółkowski, and H. Wachowska: Capacitance behaviour of the ammoxidised coal. J. Phys. Chem. Solids 65, 269 (2004).
G. Lota, B. Grzyb, H. Machnikowska, J. Machnikowski, and E. Frackowiak: Effect of nitrogen in carbon electrode on the supercapacitor performance. Chem. Phys. Lett. 404, 53 (2005).
H. Wang, K. Wang, H. Song, H. Li, S. Ji, Z. Wang, S. Li, and R. Wang: N-doped porous carbon material made from fish-bones and its highly electrocatalytic performance in the oxygen reduction reaction. RSC Adv. 5, 48965 (2015).
P. Nowicki and R. Pietrzak: Węgle aktywne wzbogacone w azot — otrzymywanie, włąsciwości I potencjalne zastosowania. In Adsorbenty i katalizatory, J. Ryczkowki, ed. (Uniwersytet Rzeszowski, Rzeszow, 2012); p. 129.
D. Hulicova-Jurcakova, M. Seredych, G.Q. Lu, and T.J. Bandosz: Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 19, 438 (2009).
H. Peng, G. Ma, K. Sun, Z. Zhang, Q. Yang, and Z. Lei: Nitrogen-doped interconnected carbon nanosheets from pomelo mesocarps for high performance supercapacitors. Electrochim. Acta 190, 862 (2016).
L. Wang and R.T. Yang: Hydrogen storage properties of N-doped microporous carbon. J. Phys. Chem. C 113, 21883 (2009).
V.J. Watson, C. Nieto Delgado, and B.E. Logan: Improvement of activated carbons as oxygen reduction catalysts in neutral solutions by ammonia gas treatment and their performance in microbial fuel cells. J. Power Sources 242, 756 (2013).
M. Ghasemi, S. Shahgaldi, M. Ismail, B.H. Kim, Z. Yaakob, and W.R. Wan Daud: Activated carbon nanofibers as an alternative cathode catalyst to platinum in a two-chamber microbial fuel cell. Int. J. Hydrogen Energy 36, 13746 (2011).
X. Yang, W. Zou, Y. Su, Y. Zhu, H. Jiang, J. Shen, and C. Li: Activated nitrogen-doped carbon nanofibers with hierarchical pore as efficient oxygen reduction reaction catalyst for microbial fuel cells. J. Power Sources 266, 36 (2014).
R. Czerw, M. Terrones, J.C. Charlier, X. Blase, B. Foley, R. Kamalakaran, N. Grobert, H. Terrones, D. Tekleab, P.M. Ajayan, W. Blau, M. Rühle, and D.L. Carroll: Identification of electron donor states in N-doped carbon nanotubes. Nano Lett. 1, 457 (2001).
K. Gong, F. Du, Z. Xia, M. Durstock, and L. Dai: Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323, 760 (2009).
G. Wu, N.H. Mack, W. Gao, S. Ma, R. Zhong, J. Han, J.K. Baldwin, and P. Zelenay: Nitrogen-doped graphene-rich catalysts derived from heteroatom polymers for oxygen reduction in nonaqueous lithium–O2 battery cathodes. ACS Nano 6, 9764 (2012).
S. Wang , E. Iyyamperumal , A. Roy, Y. Xue, D. Yu, and L. Dai: Vertically aligned BCN nanotubes as efficient metal-free electrocatalysts for the oxygen reduction reaction: A synergetic effect by Co-doping with boron and nitrogen. Angew. Chem., Int. Ed. 50, 11756 (2011).
J. Shui, M. Wang, F. Du, and L. Dai: N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 1, e1400129 (2015).
Q. Wei, X. Tong, G. Zhang, J. Qiao, Q. Gong, and S. Sun: Nitrogen-doped carbon nanotube and graphene materials for oxygen reduction reactions. Catalysis 5, 1574 (2015).
L. Feng, Y. Chen, and L. Chen: Easy-to-operate and low-temperature synthesis of gram-scale nitrogen-doped graphene and its application as cathode catalyst in microbial fuel cells. ACS Nano 5, 9611 (2011).
S. Wang, L. Zhang, Z. Xia, A. Roy, D.W. Chang, J-B. Baek, and L. Dai: BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 51, 4209 (2012).
Y. Hu, H. Liu, Q. Ke, and J. Wang: Effects of nitrogen doping on supercapacitor performance of a mesoporous carbon electrode produced by a hydrothermal soft-templating process. J. Mater. Chem. A 2, 11753 (2014).
F.L. Braghiroli, V. Fierro, M.T. Izquierdo, J. Parmentier, A. Pizzi, L. Delmotte, P. Fioux, and A. Celzard: High surface—Highly N-doped carbons from hydrothermally treated tannin. Ind. Crop. Prod. 66, 282 (2015).
J. Duan , H. Fan, and W. Shen: Nitrogen-doped carbon materials prepared from polyurethane foams. ChemistrySelect 1, 3204 (2016).
W. Li, D. Chen, Z. Li, Y. Shi, Y. Wan, G. Wang, Z. Jiang, and D. Zhao: Nitrogen-containing carbon spheres with very large uniform mesopores: The superior electrode materials for EDLC in organic electrolyte. Carbon 45, 1757 (2007).
D. Hulicova, M. Kodama, and H. Hatori: Electrochemical performance of nitrogen-enriched carbons in aqueous and non-aqueous supercapacitors. Chem. Mater. 18, 2318 (2006).
E. Pollak, G. Salitra, A. Soffer, and D. Aurbach: On the reaction of oxygen with nitrogen-containing and nitrogen-free carbons. Carbon 44, 3302 (2006).
H.M. Jeong, J.W. Lee, W.H. Shin, Y.J. Choi, H.J. Shin, J.K. Kang, and J.W. Choi: Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 11, 2472 (2011).
Y. Wang, Y. Shao, D.W. Matson, J. Li, and Y. Lin: Nitrogen-doped graphene and its application in electrochemical bisensing. ACS Nano 4, 1790 (2010).
A. Ben Belgacem, I. Hinkov, S.B. Yahia, O. Brinza, and S. Farhat: Arc discharge boron nitrogen doping of carbon nanotubes. Mater. Today Commun. 8, 183 (2016).
M. Kruk, K.M. Kohlhaas, B. Dufour, E.B. Celer, M. Jaroniec, K. Matyjaszewski, R.S. Ruoff, and T. Kowalewski: Partially graphitic, high-surface-area mesoporous carbons from polyacrylonitrile templated by ordered and disordered mesoporous silicas. Microporous Mesoporous Mater. 102, 178 (2007).
A. Lu, A. Kiefer, W. Schmidt, and F. Schüth: Synthesis of polyacrylonitrile-based ordered mesoporous carbon with tunable pore structures. Chem. Mater. 16, 100 (2004).
J. Machnikowski, B. Grzyb, H. Machnikowska, and J.V. Weber: Surface chemistry of porous carbons from N-polymers and their blends with pitch. Microporous Mesoporous Mater. 82, 113 (2005).
E. Raymundo-Piñero, D. Cazorla-Amorós, and A. Linares-Solano: The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibres. Carbon 41, 1925 (2003).
K. Cong, M. Radtke, S. Stumpf, B. Schröter, D.G. McMillan, M. Rettenmayr, and A. Ignaszak: Electrochemical stability of the polymer-derived nitrogen-doped carbon: An elusive goal? Mater. Renew. Sustain. Energy 4, 1 (2015).
M. Trchová, E.N. Konyushenko, J. Stejskal, J. Kovářová, and G. Ćirić-Marjanović: The conversion of polyaniline nanotubes to nitrogen-containing carbon nanotubes and their comparison with multi-walled carbon nanotubes. Polym. Degrad. Stab. 94, 929 (2009).
X. Yang, D. Wu, X. Chen, and R. Fu: Nitrogen-enriched nanocarbons with a 3-d continuous mesopore structure from polyacrylonitrile for supercapacitor application. J. Phys. Chem. C 114, 8581 (2010).
A.B. Fuertes and T.A. Centeno: Mesoporous carbons with graphitic structures fabricated by using porous silica materials as templates and iron-impregnated polypyrrole as precursor. J. Mater. Chem. 15, 1079 (2005).
C-M. Yang, C. Weidenthaler, B. Spliethoff, M. Mayanna, and F. Schüth: Facile template synthesis of ordered mesoporous carbon with polypyrrole as carbon precursor. Chem. Mater. 17, 355 (2005).
G. Nam, J. Park, S.T. Kim, D-b. Shin, N. Park, Y. Kim, J-S. Lee, and J. Cho: Metal-free ketjenblack incorporated nitrogen-doped carbon sheets derived from gelatin as oxygen reduction catalysts. Nano Lett. 14, 1870 (2014).
Z. Schnepp, Y. Zhang, M.J. Hollamby, B.R. Pauw, M. Tanaka, Y. Matsushita, and Y. Sakka: Doped-carbon electrocatalysts with trimodal porosity from a homogeneous polypeptide gel. J. Mater. Chem. A 1, 13576 (2013).
D-W. Lee, M-H. Jin, D. Oh, S-W. Lee, and J-S. Park: Straightforward synthesis of hierarchically porous nitrogen-doped carbon via pyrolysis of chitosan/urea/KOH mixtures and its application as a support for formic acid dehydrogenation catalysts. ACS Sustainable Chem. Eng. 5, 9935 (2017).
B. Wang, S. Li, X. Wu, J. Liu, and J. Chen: Biomass chitin-derived honeycomb-like nitrogen-doped carbon/graphene nanosheet networks for applications in efficient oxygen reduction and robust lithium storage. J. Mater. Chem. A 4, 11789 (2016).
M. Thommes, K. Kaneko, A.V. Neimark, J.P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, and K.S.W. Sing: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015).
Z.H. Sheng, L. Shao, J.J. Chen, W.J. Bao, F.B. Wang, and X.H. Xia: Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 5, 4350 (2011).
L. Lai, J.R. Potts, D. Zhan, L. Wang, C.K. Poh, C. Tang, H. Gong, Z. Shen, J. Lin, and R.S. Ruoff: Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 5, 7936 (2012).
N. Tachibana, S. Ikeda, Y. Yukawa, and M. Kawaguchi: Highly porous nitrogen-doped carbon nanoparticles synthesized via simple thermal treatment and their electrocatalytic activity for oxygen reduction reaction. Carbon 115, 515 (2017).
X. Wang, X. Li, L. Zhang, Y. Yoon, P.K. Weber, H. Wang, J. Guo, and H. Dai: N-doping of graphene through electrothermal reactions with ammonia. Science 324, 768 (2009).
ACKNOWLEDGMENT
This work was carried out as a result of the research Project No. 2014/15/N/ST8/03399 financed by the National Science Center (Poland).
Author information
Authors and Affiliations
Corresponding author
Supplementary Material
Rights and permissions
About this article
Cite this article
Ilnicka, A., Lukaszewicz, J.P., Shimanoe, K. et al. Urea treatment of nitrogen-doped carbon leads to enhanced performance for the oxygen reduction reaction. Journal of Materials Research 33, 1612–1624 (2018). https://doi.org/10.1557/jmr.2018.116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2018.116