Abstract
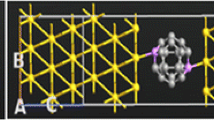
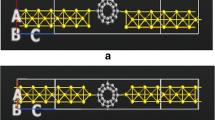
In this paper, we determine the electronic transport properties of Au–C20–Au molecular system under finite bias voltage using the non-equilibrium Green function and the density functional theory, along its localized pseudo atomic orbitals. Our aim is to peruse the various nanometer-scale transport properties and eventually predict the overall quantum transport behavior of this organic mesoscopic system. We investigate the density of states, transmission spectrum, molecular orbitals, current–voltage characteristics, rectification ratio, and differential conductance characteristics at discrete bias voltages to get the insight about various transport phenomena. The observed results elucidate that the quantum tunneling causes the electron transport in this molecular bridge and becomes prominent due to strong mechanical interactive coupling between the molecule and the electrodes having low HOMO–LUMO (highest occupied molecular orbital–lowest unoccupied molecular orbital) gap of 0.55 eV. We conclude that Au–C20–Au device exhibited metallic nature forming the current coulomb staircase with transition points at ±1 V and the quantum conductance of order 2G0 at low bias voltages.














Similar content being viewed by others
References
R.P. Feynman: There’s plenty of room at the bottom. Caltech Engg. Sci. 23 (5), 22 (1960).
M. Kaur, R.S. Sawhney, and D. Engles: Contemplating transport characteristics by augmenting the length of molecule. J. Multiscale Modell. 5 (3), 1350010 (2013).
T. Rakshit, G.C. Liang, A.W. Ghosh, and S. Datta: Silicon-based molecular electronics. Nano Lett. 4, 1803 (2004).
G.C. Liang and A.W. Ghosh: Identifying contact effects in electronic conduction through C60 on silicon. Phys. Rev. Lett. 95, 076403 (2005).
B. Muralidharan, A.W. Ghosh, and S. Datta: Probing electronic excitations in molecular conduction. Phys. Rev. B: Condens. Matter Mater. Phys. 73, 155410 (2006).
J. Chen, W. Wang, M.A. Reed, A.M. Rawlett, D.W. Price, and J.M. Tour: Room-temperature negative differential resistance in nanoscale molecular junctions. Appl. Phys. Lett. 77 (8), 1224 (2000).
S. Datta: Electronic Transport in Mesoscopic Systems, 1st ed. (Cambridge Univ. Press, Cambridge, England, 1995); pp. 266–268.
P.S. Damle, A.W. Ghosh, and S. Datta: From molecules to metallic wires: Unified description of molecular conduction. Phys. Rev. B: Condens. Matter Mater. Phys. 64, 201403 (2001).
Z. Yang, L. Wan, Y. Yu, Y. Wei, and J. Wang: Electron transport through Al–ZnO–Al: An ab initio calculation. J. Appl. Phys. 108, 033704 (2010).
W. Wang, T. Lee, and M.A. Reed: Mechanism of electron conduction in self-assembled alkanethiol monolayer devices. Phys. Rev. B: Condens. Matter Mater. Phys. 68, 035416 (2003).
S. Ke, H.U. Baranger, and W. Yang: Contact atomic structure and electron transport through molecules. J. Chem. Phys. 122, 074704 (2005).
M. Kaur, R.S. Sawhney, and D. Engles: Perusing quantum transport through geometric gold electrodes in flexible electronics. Quantum Matter 4 (2), 182 (2015).
D.Q. Andrews, R. Cohen, R.P.V. Duyne, and M.A. Ratner: Single molecule electron transport junctions: Charging and geometric effects on conductance. J. Chem. Phys. 125, 174718 (2006).
Y.C. Choi, W.Y. Kim, K. Park, P. Tarakeshwar, K.S. Kim, T. Kim, and J.Y. Lee: Role of molecular orbitals of the benzene in electronic nanodevices. J. Chem. Phys. 122, 094706 (2005).
M. Kaur and R.S. Sawhney: Anatomizing electronic transport through saturated alkane molecule with disparate terminal elements. J. Multiscale Modell. 4 (3), 1250011 (2012).
H.W. Kroto, J.R. Heath, S.C. O’Brien, R.F. Curl, and R.E. Smalley: C60: Buckminsterfullerene. Nature 318, 162, (1985).
W. Krätschmer, L.D. Lamb, K. Fostiropoulos, and D.R. Huffman: Solid C60: A new form of carbon. Nature 347, 354 (1990).
D.M. Guldi and N. Martín: Fullerenes: From Synthesis to Optoelectronic Properties (Kluwer Academic Publishers, Dordrecht, The Netherlands, 2002); pp. 121–135.
G. Zheng, S. Irle, and K. Morokuma: Performance of the DFTB method in comparison to DFT and semiempirical methods for geometries and energies of C20–C86 fullerene isomers. Chem. Phys. Lett. 412, 210 (2005).
B. Paulus: Electronic and structural properties of the cage-like molecules C20 to C36. Phys. Chem. Chem. Phys. 5, 3364 (2003).
M. Bendikovand, F. Wudl, and D.F. Perepichka: Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives: The brick and mortar of organic electronics. Chem. Rev. 104, 4891 (2004).
A. Rassat: Chirality and symmetry aspects of spheroarenes, including fullerenes. Chirality 13, 395 (2001).
A. Lappas, K. Prassides, K. Vavekis, D. Arcon, R. Blinc, P. Cevc, A. Amato, R. Feyerherm, F.N. Gygax, and A. Schenck: Spontaneous magnetic ordering in the fullerene charge-transfer salt (TDAE) C60. Science, 267 (5205), 1799 (1995).
A.F. Hebard, M.J. Rosseinsky, R.C. Haddon, D.W. Murphy, S.H. Glarum, T.T.M. Palstra, A.P. Ramirez, and A.R. Kortan: Superconductivity at 18 K in potassium-doped C60. Nature 350, 600 (1991).
L. Echegoyenand and L.E. Echegoyen: Electrochemistry of fullerenes and their derivatives. Acc. Chem. Res. 31, 593 (1998).
D.M. Guldi: Fullerenes: Three dimensional electron acceptor materials. Chem. Commun. 5, 321 (2000).
H. Prinzbach, A. Weiler, P. Landenberger, F. Wahl, L.T. Scott, M. Gelmont, D. Olevano, and B.V. Issendorff: Gas-phase production and photoelectron spectroscopy of the smallest fullerene, C20. Nature 407, 60 (2000).
K.D. Sattler: Handbook of Nanophysics: Clusters and Fullerenes (CRC Press, Boca Raton, 2009); pp. 28–36.
C. Roland, B. Larade, J. Taylor, and H. Guo: Ab initio I–V characteristics of short C20 chains. Phys. Rev. B: Condens. Matter Mater. Phys. 65, 041401(R) (2001).
T. Yamamoto, K. Watanabe, and S. Watanabe: Electronic transport in fullerene C20 bridge assisted by molecular vibrations. Phys. Rev. Lett. 95, 065501 (2005).
Y.P. An, C.L. Yang, M.S. Wang, X.G. Ma, and D.H. Wang: First-principles study of structure and quantum transport properties of C20 fullerene. J. Chem. Phys. 131, 024311 (2009).
Y.P. An, C.L. Yang, M.S. Wang, X.G. Ma, and D.H. Wang: First-principles study of transport properties of endohedral Li@C20 metallofullerene. Curr. Appl. Phys. 10, 260 (2010).
L.H. Wang, Y. Guo, C.F. Tian, X.P. Song, and B.J. Ding: Effect of the indices of crystal plane of gold electrodes on the transport properties of C20 fullerene. J. Appl. Phys. 107, 103702 (2010).
G. Ji, D. Li, C. Fang, Y. Xu, Y. Zhai, B. Cui, and D. Liu: Effect of contact interface configuration on electronic transport in (C20)2-based molecular junctions. Phys. Lett. A 376, 773, (2012).
V.K. Ivanov, G. Yu Kashenock, R.G. Polozkov, and A.V. Solov’yov: Photoionization cross sections of the fullerenes C20 and C60 calculated in a simple spherical model. J. Phys. B: At., Mol. Opt. Phys. 34, L669 (2001).
D. Xiang, H. Jeong, D. Kim, T. Lee, Y. Cheng, Q. Wang, and D. Mayer: Three-terminal single-molecule junctions formed by mechanically controllable break junctions with side gating. Nano Lett. 13, 2809 (2013).
M. Kaur, R.S. Sawhney, and D. Engles: To evince pure C24 as superconductoring mechanically controllable break junction configuration. In 2013 International Conference on Advanced Nanomaterials and Emerging Engineering Technologies (ICANMEET) (IEEE: Chennai, 2013); pp. 426–430.
A. Goel, J.B. Howard, and J.B. Vander Sande: Size analysis of single fullerene molecules by electron microscopy. Carbon 42, 1907 (2004).
T.E. Karakasidis and C.A. Charitidis: Multiscale modeling in nanomaterials science. Mater. Sci. Eng., C 27 (5), 1082 (2007).
A. Ferreira and S.S. Aphale: A survey of modeling and control techniques for micro- and nanoelectromechanical systems. IEEE Trans. Syst. Man Cybern. C Appl. Rev. 41 (3), 350 (2011).
M. Brandbyge, J.L. Mozos, P. Ordejon, J. Taylor, and K. Stokbro: Density-functional method for nonequilibrium electron transport. Phys. Rev. B: Condens. Matter Mater. Phys. 65, 165401 (2002).
K. Stokbro: First-principles modeling of electron transport. J. Phys.: Condens. Matter 20, 064216 (2008).
M.Y. Xue, S. Datta, and M.A. Ratner: First-principles based matrix-Green’s function approach to molecular electronic devices: General formalism. Chem. Phys. 281, 151 (2002).
Atomistix Tool Kit Manual version 12.2.0 (Copyright QuantumWise 2008–2015).
J.P. Perdew, K. Burke, and M. Ernzerhof: Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Y. Cho, W.Y. Kim, and K.S. Kim: Effect of electrodes on electronic transport of molecular electronic devices. J. Phys. Chem. A 113, 4100 (2009).
Y. Kim, J.T. Kheli, P.A. Schultz, and W.A. Goddard: First-principles approach to the charge-transport characteristics of monolayer molecular-electronics devices: Application to hexanedithiolate devices. Phys. Rev. B: Condens. Matter Mater. Phys. 73, 235419 (2006).
M. Strange, S. Kristensen, K.S. Thygesen, and K.W. Jacobsen: Benchmark density functional theory calculations for nanoscale conductance. J. Chem. Phys. 128, 114714 (2008).
N. Troullier and J.L. Martins: Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B: Condens. Matter Mater. Phys. 43, 1993 (1991).
ATK-tutorials: Why are so many k-points needed in the transport direction in a device calculation? (2014).
A detailed discussion is given in the supporting information.
J. Luo, Z.Q. Xue, W.M. Liu, J.L. Wu, and Z.Q. Yang: Koopmans’ theorem for large molecular systems within density functional theory. J. Phys. Chem. A 110, 12005 (2006).
F.A. Gianturco, G.Y. Kashenock, R.R. Lucchese, and N. Sanna: Low-energy resonant structures in electron scattering from C20 fullerene. J. Chem. Phys. 116, 2811 (2002).
R. Cohen, K. Stokbro, J.M.L. Martin, and M.A. Ratner: Charge transport in conjugated aromatic molecular junctions: Molecular conjugation and molecule–electrode coupling. J. Phys. Chem. C 111, 14893 (2007).
H. Morkoc: Advanced Semiconductors and Organic Nano-techniques (Academic Press, New York, 2003).
H. Song, M.A. Reed, and T. Lee: Single molecule electronic devices. Adv. Mater. 23, 1583 (2011).
C. Durkan: Current at the Nanoscale (Imperial College Press, London, 2007); pp. 10–12.
B.N. Taylor and P.J. Mohr: Codata Value: Conductance quantum (2010). Available at: http://physics.nist.gov/cgi-bin/cuu/Value?conqu2e2sh. Retrieved 2016-02-08.
B.J. van Wees, H. van Houten, C.W.J. Beenakker, J.G. Williamson, L.P. Kouwenhoven, D. van der Marel, and C.T. Foxon: Quantized conductance of point contacts in a two-dimensional electron gas. Phys. Rev. Lett. 60, 848 (1988).
W. Liang, M.P. Shores, M. Bockrath, J.R. Long, and H. Park: Kondo resonance in a single-molecule transistor. Nature 417, 725 (2002).
J. Park, A.N. Pasupathy, J.I. Goldsmith, C. Chang, Y. Yaish, J.R. Petta, M. Rinkoski, J.P. Sethna, H.D. Abrunã, P.L. McEuen, and D.C. Ralph: Coulomb blockade and the Kondo effect in single-atom transistors. Nature 417, 722 (2002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kaur, M., Sawhney, R.S. & Engles, D. Non-equilibrium tunneling through Au–C20–Au molecular bridge using density functional theory–non-equilibrium Green function approach. Journal of Materials Research 31, 2025–2034 (2016). https://doi.org/10.1557/jmr.2016.170
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2016.170