Abstract
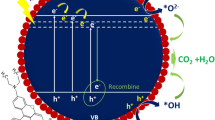
Tungsten oxide (WO3−x) nanomaterials with controlled morphology and composition were fabricated by thermal evaporation of WO3 and S powders at different temperatures in a vacuum tube furnace. At 850 °C the obtained green particle is still of the same monoclinic WO3 phase as that of the starting powder. At a temperature between 900 and 1100 °C, the resultant dark-blue products are particle-like clusters composed of numerous monoclinic WO2.90 short nanorods, but the clusters became looser and the nanorods grew somewhat longer as the temperature increased. At a temperature between 1150 and 1250 °C, elongated and thoroughly separate purple-red monoclinic W18O49 nanorods were obtained. The growth of the prepared WO3−x nanomaterials was controlled by a gas–solid mechanism. Their photocatalytic degradation on organic contaminants was evaluated by decomposing methylene blue (MB) in aqueous phase under sunlight, in which WO3 particles presented higher photocatalytic activity than its oxygen-deficient counterparts, WO2.90 and W18O49. But the W18O49 nanorods had higher adsorption ability to MB in all the samples.








Similar content being viewed by others
References
M. Boulova, A. Gaskov, and G. Lucazeau: Tungsten oxide reactivity versus CH4, CO, and NO2 molecules studied by Raman spectroscopy. Sens. Actuators, B 81, 99 (2001).
J.L. Solis, S. Saukko, L. Kish, C.G. Granqvist, and V. Lantto: Semiconductor gas sensors based on nanostructured tungsten oxide. Thin Solid Films 391, 255 (2001).
J. Polleux, N. Pinna, M. Antonietti, and M. Niederberger: Growth and assembly of crystalline tungsten oxide nanostructures assisted by bioligation. J. Am. Chem. Soc. 127, 15595 (2005).
G.C. Xi, J.H. Ye, Q. Ma, N. Su, H. Bai, and C. Wang: In situ growth of metal particles on 3D urchin-like WO3 nanostructures. J. Am. Chem. Soc. 134, 6508 (2012).
B. Rausch, M.D. Symes, G. Chisholm, and L. Cronin: Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting. Science 345, 1326 (2014).
J. Kim, C.W. Lee, and W. Choi: Platinized WO3 as an environmental photocatalyst that generates OH radicals under visible light. Environ. Sci. Technol. 44, 6849 (2010).
I.M. Szilagyi, B. Forizs, O. Rosseler, A. Szegedi, P. Nemeth, P. Kiraly, G. Tarkanyi, B. Vajna, K. Varga-Josepovits, K. Laszlo, A.L. Toth, P. Baranyai, and M. Leskela: WO3 photocatalysts: Influence of structure and composition. J. Catal. 294, 119 (2012).
X.L. Li, T.J. Lou, X.M. Sun, and Y.D. Li: Highly sensitive WO3 hollow-sphere gas sensors. Inorg. Chem. 43, 5442 (2004).
H.G. Wei, X.R. Yan, S.J. Wu, Z.P. Luo, S.Y. Wei, and Z.H. Guo: Electropolymerized polyaniline stabilized tungsten oxide nanocomposite films: Electrochromic behavior and electrochemical energy storage. J. Phys. Chem. C 116, 25052 (2012).
W.T. Wu, J.J. Wu, and J.S. Chen: Resistive switching behavior and multiple transmittance states in solution-processed tungsten oxide. ACS Appl. Mater. Interfaces 3, 2616 (2011).
J.Q. Wen, X. Li, W. Liu, Y.P. Fang, J. Xie, and Y.H. Xu: Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chin. J. Catal. 36, 2049 (2015).
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, and Y. Taga: Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269 (2001).
L. Liu and X.B. Chen: Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 114, 9890 (2014).
X.B. Chen, L. Liu, and F.Q. Huang: Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 44, 1861 (2015).
S.W. Cao, J.X. Low, J.G. Yu, and M. Jaroniec: Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 27, 2150 (2015).
Z.Y. Wang, W. Guan, Y.J. Sun, F. Dong, Y. Zhou, and W.K. Ho: Water-assisted production of honeycomb-like g-C3N4 with ultralong carrier lifetime and outstanding photocatalytic activity. Nanoscale 7, 2471 (2015).
C.M. Foeldvary and L. Wojnarovits: The effect of high-energy radiation on aqueous solution of Acid Red 1 textile dye. Radiat. Phys. Chem. 76, 1485 (2007).
Y.P. Chen, S.Y. Liu, H.Q. Yu, H. Yin, and Q.R. Li: Radiation-induced degradation of methyl orange in aqueous solutions. Chemosphere 72, 532 (2008).
M. Qamar, M.A. Gondal, and Z.H. Yamani: Synthesis of highly active nanocrystalline WO3 and its application in laser-induced photocatalytic removal of a dye from water. Catal. Commun. 10, 1980 (2009).
M.A. Rauf and S.S. Ashraf: Radiation induced degradation of dyes-an overview. J. Hazard. Mater. 166, 6 (2009).
H.B. Fu, C.S. Pan, W.Q. Yao, and Y.F. Zhu: Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 109, 22432 (2005).
A. Fujishima, X.T. Zhang, and D.A. Tryk: TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 63, 515 (2008).
G.R. Bamwenda, K. Sayama, and H. Arakawa: The effect of selected reaction parameters on the photoproduction of oxygen and hydrogen from a WO3–Fe2+–Fe3+ aqueous suspension. J. Photochem. Photobiol., A 122, 175 (1999).
L. Wan, J. Sheng, H. Chen, and Y. Xu: Different recycle behavior of Cu2+ and Fe3+ ions for phenol photodegradation over TiO2 and WO3. J. Hazard. Mater. 262, 114 (2013).
X. Li, J.G. Yu, J.X. Low, Y.P. Fang, J. Xiao, and X.B. Chen: Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 3, 2485 (2015).
I.M. Szilagyi, J. Madarasz, G. Pokol, P. Kiraly, G. Tarkanyi, S. Saukko, J. Mizsei, A.L. Toth, A. Szabo, and K. Varga-Josepovitso: Stability and controlled composition of hexagonal WO3. Chem. Mater. 20, 4116 (2008).
T.J. DeJournett and J.B. Spicer: Photoinduced silver precursor decomposition for particle modification in tungsten oxide-polymer matrix nanocomposites. J. Phys. Chem. C 118, 9820 (2014).
E.K. Papynov, V.Y. Mayorov, M.S. Palamarchuk, and V.A. Avramenko: Peculiarities of formation of phase composition, porous structure, and catalytic properties of tungsten oxide-based macroporous materials fabricated by sol–gel synthesis. Mater. Charact. 88, 42 (2014).
M. Ahmadi, R. Younesi, and M.J.F. Guinel: Synthesis of tungsten oxide nanoparticles using a hydrothermal method at ambient pressure. J. Mater. Res. 29, 1424 (2014).
C.Z. Yang, N.K. van der Laak, K.Y. Chan, and X. Zhang: Microwave-assisted microemulsion synthesis of carbon supported Pt-WO3 nanoparticles as an electrocatalyst for methanol oxidation. Electrochim. Acta 75, 262 (2012).
J.W. Qian, Z.Y. Zhao, Z.G. Shen, G.L. Zhang, Z.J. Peng, and X.L. Fu: A large scale of CuS nano-networks: Catalyst-free morphologically controllable growth and their application as efficient photocatalysts. J. Mater. Res. 30, 3746 (2015).
F.J. Wu, W. Liu, J.L. Qiu, J.Z. Li, W.Y. Zhou, Y.P. Fang, S.T. Zhang, and X. Li: Enhanced photocatalytic degradation and adsorption of methylene blue via TiO2 nanocrystals supported on graphene-like bamboo charcoal. Appl. Surf. Sci. 358, 425 (2015).
H.W. Huang, K. Liu, K. Chen, Y.L. Zhang, Y.H. Zhang, and S.C. Wang: Ce and F comodification on the crystal structure and enhanced photocatalytic activity of Bi2WO6 photocatalyst under visible light irradiation. J. Phys. Chem. C 118, 14379 (2014).
R.S. Wagner and W.C. Ellis: Vapor–liquid–solid mechanism of single crystal growth. Appl. Phys. Lett. 4, 89 (1964).
J.Y. Chen, B.J. Wiley, and Y.N. Xia: One-dimensional nanostructures of metals: Large-scale synthesis and some potential applications. Langmuir 23, 4120 (2007).
T.J. Trentler, K.M. Hickman, S.C. Geol, A.M. Viano, P.C. Gibbons, and W.E. Buhro: Solution–liquid–solid growth of crystalline III–V semiconductors: An analogy to vapor–liquid–solid growth. Science 270, 1791 (1995).
L. Palmisano, V. Augugliaro, A. Sclafani, and M. Schiavello: Activity of chromium-ion-doped titania for the dinitrogen photoreduction to ammonia and for the phenol photodegradation. J. Phys. Chem. 92, 6710 (1988).
J.M. Herrmann, J. Disdier, and P. Pichat: Effect of chromium doping on the electrical and catalytic properties of powder titania under UV and visible illumination. Chem. Phys. Lett. 108, 618 (1984).
O. Carp, C.L. Huisman, and A. Reller: Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 32, 33 (2004).
S.H. Lee, H.M. Cheong, C.E. Tracy, A. Mascarenhas, D.K. Benson, and S.K. Deb: Raman spectroscopic studies of electrochromic a-WO3. Electrochim. Acta 44, 3111 (1999).
J.W. Qian, Z.Y. Zhao, Z.G. Shen, G.L. Zhang, Z.J. Peng, and X.L. Fu: Oxide vacancies enhanced visible active photocatalytic W19O55 NMRs via strong adsorption. RSC Adv. 6, 8061 (2016).
ACKNOWLEDGMENTS
The authors would like to thank the financial support for this work from the National Natural Science Foundation of China (Grant Nos. 61274015, 11274052, and 51172030), Excellent Adviser Foundation in China University of Geosciences from the Fundamental Research Funds for the Central Universities, and Fund of State Key Laboratory of Information Photonics and Optical Communications (Beijing University of Posts and Telecommunications).
Author information
Authors and Affiliations
Corresponding authors
Supplementary Material
43578_2016_31081065_MOESM1_ESM.docx
Supplementary Materials: Synthesis of WO3−X nanomaterials with controlled morphology and composition for highly efficient photocatalysis (approximately 33.2 MB)
Rights and permissions
About this article
Cite this article
Shen, Z., Zhao, Z., Qian, J. et al. Synthesis of WO3−x nanomaterials with controlled morphology and composition for highly efficient photocatalysis. Journal of Materials Research 31, 1065–1076 (2016). https://doi.org/10.1557/jmr.2016.106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2016.106