Abstract
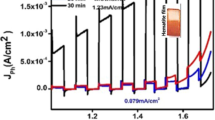
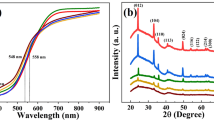
This work describes the effects of different atmospheres used during the thermal treatment of hematite films synthesized on transparent conductive substrates of fluorine-doped tin oxide by a newly reported wet chemical route assisted by microwave. The as-synthesized films were subjected to additional thermal treatment at 750 °C for 30 min in different gas flux (air, O2, and N2) to obtain a desirable phase and surface activation. A series of techniques were used to elucidate effects of each atmosphere used during the thermal treatment. The morphology of the films, as analyzed by top-view and cross-sectional scanning electron microscopy images, showed no significant changes and was composed of rods homogeneously distributed over the substrate, which covered the immersed area with a thickness between 98 and 100 nm. The photoelectrochemical response of the N2-hematite films was found to be 80 and 50% more efficient at 1.23 VRHE (reversible hydrogen electrode) than those of films produced in air and an O2 atmosphere. The photocurrent enhancement achieved by treatment in an oxygen-deficient atmosphere was attributed to the improvement of hematite catalytic activity, which produced a hematite–electrolyte interface favorable for water oxidation. Since an increase in the donor density by one order of magnitude was found for the N2-hematite films, a reduction of charge transfer resistance was expected in these films. However, the Nyquist plot analysis showed that the O2-hematite film had a lower charge transfer resistance. As a result, it is impossible to relate the photocurrent enhancement observed in N2-hematite film to electronic changes or vacancy formation, as previously reported in the literature. Indeed, by performing photoelectrochemical measurements in the presence of hole scavengers, it became clear that the major improvement caused by the oxygen-deficient atmosphere was in the catalytic activity efficiency of the hematite films for water oxidation. It was found that the oxygen-deficient atmosphere could improve the overall photoelectrochemical performance of the hematite by acting as a hole scavengers. This finding contrasts with a previous report, in which the use of an oxygen-deficient atmosphere during the phase transformation from akaganeite to hematite was found to enhance the photocurrent density by inducing an increased donor density caused by the formation of vacancies [Y. Ling et al., Angew. Chem., Int. Ed. 51, 4074 (2012)].






Similar content being viewed by others
References
M.G. Walter, E.L. Warren, J.R. McKone, S.W. Boettcher, Q. Mi, E.A. Santori, and N.S. Lewis: Solar water splitting cells. Chem. Rev. 110(11), 6446 (2010).
T.R. Cook, D.K. Dogutan, S.Y. Reece, Y. Surendranath, T.S. Teets, and D.G. Nocera: Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110(11), 6474 (2010).
M.J. Katz, S.C. Riha, N.C. Jeong, A.B.F. Martinson, O.K. Farha, and J.T. Hupp: Toward solar fuels: Water splitting with sunlight and “rust”?Coord. Chem. Rev. 256(21–22), 2521 (2012).
K. Maeda: Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol., C 12(4), 237 (2011).
C.X. Kronawitter, L. Vayssieres, S. Shen, L. Guo, D.A. Wheeler, J.Z. Zhang, B.R. Antoun, and S.S. Mao: A perspective on solar-driven water splitting with all-oxide hetero-nanostructures. Energy Environ. Sci. 4, 3889 (2011).
Á. Valdés, J. Brillet, M. Grätzel, H. Gudmundsdóttir, H.A. Hansen, H. Jónsson, P. Klüpfel, G-J. Kroes, F. Le Formal, I.C. Man, R.S. Martins, J.K. Nørskov, J. Rossmeisl, K. Sivula, A. Vojvodic, and M. Zäch: Solar hydrogen production with semiconductor metal oxides: New directions in experiment and theory. Phys. Chem. Chem. Phys. 14, 49 (2012).
R.M. Navarro, M.C. Alvarez-Galvan, J.A. Villoria de la Mano, S.M. Al-Zahrani, and J.L.G. Fierro: A framework for visible-light water splitting. Energy Environ. Sci. 3(12), 1865 (2010).
R. van de Krol, Y. Liang, and J. Schoonman: Solar hydrogen production with nanostructured metal oxides. J. Mater. Chem. 18(20), 2311 (2008).
B.D. Alexander, P.J. Kulesza, I. Rutkowska, R. Solarska, and J. Augustynski: Metal oxide photoanodes for solar hydrogen production. J. Mater. Chem. 18(20), 2298 (2008).
K. Shankar, J.I. Basham, N.K. Allam, O.K. Varghese, G.K. Mor, X. Feng, M. Paulose, J.A. Seabold, K-S. Choi, and C.A. Grimes: Recent advances in the Use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J. Phys. Chem. C 113(16), 6327 (2009).
K. Sivula, F. Le Formal, and M. Grätzel: Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 4(4), 432 (2011).
Y. Tachibana, L. Vayssieres, and J.R. Durrant: Artificial photosynthesis for solar water-splitting. Nat. Photonics 6(8), 511 (2012).
K.L. Hardee and A.J. Bard: Semiconductor electrodes. V. The application of chemically vapor deposited iron oxide films to photosensitized electrolysis. J. Electrochem. Soc. 123, 1024 (1976).
K.L. Hardee and A.J. Bard: Semiconductor electrodes. X. Photoelectrochemical behavior of several polycrystalline metal oxide electrodes in aqueous solutions. J. Electrochem. Soc. 124(2), 215 (1977).
U. Bjoerksten, J. Moser, and M. Graetzel: Photoelectrochemical studies on nanocrystalline hematite films. Chem. Mater. 6(6), 858 (1994).
F.L. Souza, K.P. Lopes, E. Longo, and E.R. Leite: The influence of the film thickness of nanostructured α-Fe2O3 on water photooxidation. Phys. Chem. Chem. Phys. 11, 1215 (2009).
F.L. Souza, K.P. Lopes, P.A.P. Nascente, and E.R. Leite: Nanostructured hematite thin films produced by spin-coating deposition solution: Application in water splitting. Sol. Energy Mater. Sol. Cells 93(3), 362 (2009).
R.H. Gonçalves, B.H.R. Lima, and E.R. Leite: Magnetite colloidal nanocrystals: A facile pathway to prepare mesoporous hematite thin films for photoelectrochemical water splitting. J. Am. Chem. Soc. 133(15), 6012 (2011).
K. Sivula, R. Zboril, F. Le Formal, R. Robert, A. Weidenkaff, J. Tucek, J. Frydrych, and M. Grätzel: Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J. Am. Chem. Soc. 132(21), 7436 (2010).
J. Brillet, M. Grätzel, and K. Sivula: Decoupling feature size and functionality in solution-processed, porous hematite electrodes for solar water splitting. Nano Lett. 10(10), 4155 (2010).
A. Kay, I. Cesar, and M. Grätzel: New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 128(49), 15714 (2006).
I. Cesar, A. Kay, J.A. Gonzalez Martinez, and M. Grätzel: Translucent thin film Fe2O3 photoanodes for efficient water splitting by sunlight: Nanostructure-directing effect of Si-doping. J. Am. Chem. Soc. 128(14), 4582 (2006).
T. Hisatomi, J. Brillet, M. Cornuz, F. Le Formal, N. Tetreault, K. Sivula, and M. Grätzel: A Ga2O3 underlayer as an isomorphic template for ultrathin hematite films toward efficient photoelectrochemical water splitting. Faraday Discuss. 155, 223 (2012).
T. Hisatomi, H. Dotan, M. Stefik, K. Sivula, A. Rothschild, M. Grätzel, and N. Mathews: Enhancement in the performance of ultrathin hematite photoanode for water splitting by an oxide underlayer. Adv. Mater. 24(20), 2699 (2012).
L. Vayssieres, N. Beermann, S.E. Lindquist, and A. Hagfeldt: Controlled aqueous chemical growth of oriented three-dimensional crystalline nanorod arrays: Application to iron(III) oxides. Chem. Mater. 13(2), 233 (2001).
N. Beermann, L. Vayssieres, S-E. Lindquist, and A. Hagfeldt: Photoelectrochemical studies of oriented nanorod thin films of hematite. J. Electrochem. Soc. 147(7), 2456 (2000).
L. Vayssieres, C. Sathe, S.M. Butorin, D.K. Shuh, J. Nordgren, and J. Guo: One-dimensional quantum-confinement effect in α-Fe2O3 ultrafine nanorod arrays. Adv. Mater. 17(19), 2320 (2005).
R. Morrish, M. Rahman, J.M.D. MacElroy, and C.A. Wolden: Activation of hematite nanorod arrays for photoelectrochemical water splitting. ChemSusChem 4(4), 474 (2011).
D.K. Bora, A. Braun, R. Erni, G. Fortunato, T. Graule, and E.C. Constable: Hydrothermal treatment of a hematite film leads to highly oriented faceted nanostructures with enhanced photocurrents. Chem. Mater. 23(8), 2051 (2011).
V.A.N. de Carvalho, R.A.S. Luz, B.H. Lima, F.N. Crespilho, E.R. Leite, and F.L. Souza: Highly oriented hematite nanorods arrays for photoelectrochemical water splitting. J. Power Sources 205, 525 (2012).
L.C. Ferraz, W.M. Carvalho, Jr., D. Criado, and F.L. Souza: Vertically oriented iron oxide films produced by hydrothermal process: Effect of thermal treatment on the physical chemical properties. ACS Appl. Mater. Interfaces 4(10), 5515 (2012).
M. Cornuz, M. Grätzel, and S. Kevin: Preferential orientation in hematite films for solar hydrogen production via water splitting. Chem. Vap. Deposition 16(10–12), 291 (2010).
R.H. Goncalves and E.R. Leite: The colloidal nanocrystal deposition process: An advanced method to prepare high performance hematite photoanodes for water splitting. Energy Environ. Sci. 7(7), 2250 (2014).
S.C. Warren, K. Voïtchovsky, H. Dotan, C.M. Leroy, M. Cornuz, F. Stellacci, C. Hébert, A. Rothschild, and M. Grätzel: Identifying champion nanostructures for solar water-splitting. Nat. Mater. 12, 842 (2013).
C.X. Kronawitter, J.R. Bakke, D.A. Wheeler, W-C. Wang, C. Chang, B.R. Antoun, J.Z. Zhang, J. Guo, S.F. Bent, S.S. Mao, and L. Vayssieres: Electron enrichment in 3d transition metal oxide hetero-nanostructures. Nano Lett. 11(9), 3855 (2011).
L. Xi, P.D. Tran, S.Y. Chiam, P.S. Bassi, W.F. Mak, H.K. Mulmudi, S.K. Batabyal, J. Barber, J.S.C. Loo, and L.H. Wong: Co3O4-Decorated hematite nanorods As an effective photoanode for solar water oxidation. J. Phys. Chem. C 116(26), 13884 (2012).
S. Shen, C.X. Kronawitter, D.A. Wheeler, P. Guo, S.A. Lindley, J. Jiang, J.Z. Zhang, L. Guo, and S.S. Mao: Physical and photoelectrochemical characterization of Ti-doped hematite photoanodes prepared by solution growth. J. Mater. Chem. A 1(46), 14498 (2013).
A. Mao, J.K. Kim, K. Shin, D.H. Wang, P.J. Yoo, G.Y. Han, and J.H. Park: Hematite modified tungsten trioxide nanoparticle photoanode for solar water oxidation. J. Power Sources 210, 32 (2012).
L. Steier, I. Herraiz-Cardona, S. Gimenez, F. Fabregat-Santiago, J. Bisquert, S.D. Tilley, and M. Grätzel: Understanding the role of underlayers and overlayers in thin film hematite photoanodes. Adv. Funct. Mater. 24(48), 7681 (2014).
W.M. Carvalho, Jr. and F.L. Souza: Recent advances on solar water splitting using hematite nanorod film produced by purpose-built material methods. J. Mater. Res. 29(01), 16 (2014).
R.H. Goncalves and E.R. Leite: Nanostructural characterization of mesoporous hematite thin film photoanode used for water splitting. J. Mater. Res. 29(01), 47 (2014).
I. Cesar, K. Sivula, A. Kay, R. Zboril, and M. Grätzel: Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J. Phys. Chem. C 113(2), 772 (2008).
J. Gan, X. Lu, and Y. Tong: Towards highly efficient photoanodes: Boosting sunlight-driven semiconductor nanomaterials for water oxidation. Nanoscale 6(13), 7142 (2014).
Y. Ling, G. Wang, J. Reddy, C. Wang, J.Z. Zhang, and Y. Li: The influence of oxygen content on the thermal activation of hematite nanowires. Angew. Chem., Int. Ed. 51(17), 4074 (2012).
M. Forster, R.J. Potter, Y. Ling, Y. Yang, D.R. Klug, Y. Li, and A.J. Cowan: Oxygen deficient α-Fe2O3 photoelectrodes: A balance between enhanced electrical properties and trap-mediated losses. Chem. Sci. 6(7), 4009 (2015).
A.M. Xavier, F.F. Ferreira, and F.L. Souza: Morphological and structural evolution from akaganeite to hematite of nanorods monitored by ex situ synchrotron X-ray powder diffraction. RSC Adv. 4(34), 17753 (2014).
D.M. Cunha and F.L. Souza: Facile synthetic route for producing one-dimensional zinc oxide nanoflowers and characterization of their optical properties. J. Alloys Compd. 577, 158 (2013).
T. Ami and M. Suzuki: MOCVD growth of (100)-oriented CeO2 thin films on hydrogen-terminated Si(100) substrates. Mater. Sci. Eng., B 54(1–2), 84 (1998).
S. Shen, C.X. Kronawitter, J. Jiang, S.S. Mao, and L. Guo: Surface tuning for promoted charge transfer in hematite nanorod arrays as water-splitting photoanodes. Nano Res. 5(5), 327 (2012).
W. Rasband: ImageJ (Bethesda, MD: National Institutes of Health, 2015).
M. Anpo and M. Takeuchi: The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 216(1–2), 505 (2003).
X. Pan, M-Q. Yang, X. Fu, N. Zhang, and Y-J. Xu: Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 5(9), 3601 (2013).
R. van de Krol and M. Gratzel: Photoelectrochemical Hydrogen Production (New York: Springer Science, 2012).
T. Lopes, L. Andrade, F. Le Formal, M. Gratzel, K. Sivula, and A. Mendes: Hematite photoelectrodes for water splitting: Evaluation of the role of film thickness by impedance spectroscopy. Phys. Chem. Chem. Phys. 16(31), 16515 (2014).
K. Sivula: Metal oxide photoelectrodes for solar fuel production, surface traps, and catalysis. J. Phys. Chem. Lett. 4(10), 1624–1633 (2013).
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the Brazilian agencies of FAPESP (Grants 2011/19924-2, 2013/05471-7, 2014/50516-6 and 2014/11736-0), CAPES, CNPq (Grant No. 473669/2012-9), CEM-UFABC and CDMF (Grant No. 2013/07296-2)
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper has been selected as an Invited Feature Paper.
Supplementary Material
43578_2015_30233595_MOESM1_ESM.docx
Supporting Information for Enhancing water oxidation efficiency of hematite thin films by using oxygen deficient atmosphere (approximately 35.8 KB)
Rights and permissions
About this article
Cite this article
Freitas, A.L.M., Carvalho, W.M. & Souza, F.L. Enhanced water oxidation efficiency of hematite thin films by oxygen-deficient atmosphere. Journal of Materials Research 30, 3595–3604 (2015). https://doi.org/10.1557/jmr.2015.353
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.353