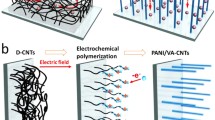
Abstract
Developing high energy density supercapacitors is of great importance to the transportation, consumer electronics, and micro-grid energy storage sectors. Recently, the development of high voltage organic electrolyte based supercapacitor devices has been gaining much attention. Among them, there is an on-going intense interest in investigating high capacity lithium ion storage anode materials in hybrid supercapacitors. However, developing high capacity cathode materials for high voltage organic electrolyte supercapacitor devices is rarely investigated. The low electrical double layer capacitances of carbon cathode electrodes, which are widely used in current supercapacitor devices, are often the limiting bottleneck. In this contribution, we investigated the electrochemical energy storage behavior of a polyaniline (PANI)-single wall carbon nanotube (SWCNT) composite material in an organic electrolyte as a supercapacitor cathode. The PANI-SWCNT composite exhibits a high specific capacitance of 503 F/g, of which 58.8% of the total capacitance is attributed to the pseudocapacitive and electrical double layer energy storage. The cycling stability of the PANI-SWCNT composite could be further improved by polydopamine (PDA) modification. The PDA with strong adhesion properties is able to prevent mechanical degradation. The PDA modified PANI-SWCNT shows excellent stability with only 5% degradation after 2000 cycles.







Similar content being viewed by others
References
C. Liu, F. Li, L.P. Ma, and H.M. Cheng: Advanced materials for energy storage. Adv. Mater. 22(8), E28 (2010).
A. Du Pasquier, I. Plitz, S. Menocal, and G. Amatucci: A comparative study of Li-ion battery, supercapacitor and nonaqueous asymmetric hybrid devices for automotive applications. J. Power Sources 115(1), 171 (2003).
A. Burke: R&D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 53(3), 1083 (2007).
K. Naoi, S. Ishimoto, J-i. Miyamoto, and W. Naoi: Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 5(11), 9363 (2012).
V. Aravindan, J. Gnanaraj, Y-S. Lee, and S. Madhavi: Insertion-type electrodes for nonaqueous Li-ion capacitors. Chem. Rev. 114(23), 11619 (2014).
L.L. Zhang and X. Zhao: Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 38(9), 2520 (2009).
V. Augustyn, P. Simon, and B. Dunn: Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7(5), 1597 (2014).
B. Kim, H. Chung, and W. Kim: High-performance supercapacitors based on vertically aligned carbon nanotubes and nonaqueous electrolytes. Nanotechnology 23(15), 155401 (2012).
V. Gupta and N. Miura: Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 52(4), 1721 (2006).
K. Wang, P. Zhao, X. Zhou, H. Wu, and Z. Wei: Flexible supercapacitors based on cloth-supported electrodes of conducting polymer nanowire array/SWCNT composites. J. Mater. Chem. 21(41), 16373 (2011).
A. Sumboja, U.M. Tefashe, G. Wittstock, and P.S. Lee: Investigation of charge transfer kinetics of polyaniline supercapacitor electrodes by scanning electrochemical microscopy. Adv. Mater. Interfaces 2(1), 1400154 (2015).
E. Song and J-W. Choi: Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 3(3), 498 (2013).
J. Yan, L. Yang, M. Cui, X. Wang, K.J. Chee, V.C. Nguyen, V. Kumar, A. Sumboja, M. Wang, and P.S. Lee: Aniline tetramer-graphene oxide composites for high performance supercapacitors. Adv. Energy Mater. 4(18), 1400781 (2014).
H. Lee, S.M. Dellatore, W.M. Miller, and P.B. Messersmith: Mussel-inspired surface chemistry for multifunctional coatings. Science 318(5849), 426 (2007).
J. Yan, L. Yang, M.F. Lin, J. Ma, X. Lu, and P.S. Lee: Polydopamine spheres as active templates for convenient synthesis of various nanostructures. Small 9(4), 596 (2013).
J.H. Hafner, M.J. Bronikowski, B.R. Azamian, P. Nikolaev, A.G. Rinzler, D.T. Colbert, K.A. Smith, and R.E. Smalley: Catalytic growth of single-wall carbon nanotubes from metal particles. Chem. Phys. Lett. 296(1–2), 195 (1998).
M.E. Lynge, R. van der Westen, A. Postma, and B. Städler: Polydopamine—a nature-inspired polymer coating for biomedical science. Nanoscale 3(12), 4916 (2011).
T. Abdiryim, Z. Xiao-Gang, and R. Jamal: Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 90(2–3), 367 (2005).
M. Trchová, I. Šeděnková, E. Tobolková, and J. Stejskal: FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stab. 86(1), 179 (2004).
W. Zheng, M. Angelopoulos, A.J. Epstein, and A.G. MacDiarmid: Experimental evidence for hydrogen bonding in polyaniline: mechanism of aggregate formation and dependency on oxidation state. Macromolecules 30(10), 2953 (1997).
R.A. Zangmeister, T.A. Morris, and M.J. Tarlov: Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 29(27), 8619 (2013).
C. Peng, D. Hu, and G.Z. Chen: Theoretical specific capacitance based on charge storage mechanisms of conducting polymers: Comment on ‘Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties’. Chem. Commun. 47(14), 4105 (2011).
K. Zhang, L.L. Zhang, X.S. Zhao, and J. Wu: Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 22(4), 1392 (2010).
C-C. Hu and J-Y. Lin: Effects of the loading and polymerization temperature on the capacitive performance of polyaniline in NaNO3. Electrochim. Acta 47(25), 4055 (2002).
S.R. Sivakkumar, J-S. Oh, and D-W. Kim: Polyaniline nanofibres as a cathode material for rechargeable lithium-polymer cells assembled with gel polymer electrolyte. J. Power Sources 163(1), 573 (2006).
S. Ardizzone, G. Fregonara, and S. Trasatti: INNER and outer active surface of RUO2 electrodes. Electrochim. Acta 35(1), 263 (1990).
X. Petrissans, A. Betard, D. Giaume, P. Barboux, B. Dunn, L. Sicard, and J.Y. Piquemal: Solution synthesis of nanometric layered cobalt oxides for electrochemical applications. Electrochim. Acta 66, 306 (2012).
M. Sathiya, A.S. Prakash, K. Ramesha, J.M. Tarascon, and A.K. Shukla: V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 133(40), 16291 (2011).
G.A. Snook, P. Kao, and A.S. Best: Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 196(1), 1 (2011).
W.J. Albery, Z. Chen, B.R. Horrocks, A.R. Mount, P.J. Wilson, D. Bloor, A.T. Monkman, and C.M. Elliott: Spectroscopic and electrochemical studies of charge transfer in modified electrodes. Faraday Discuss. Chem. Soc. 88, 247 (1989).
B.E. Conway: Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications (Kluwer Academic/Plenum, New York, 1999).
X. Wang, W.S. Liu, X. Lu, and P.S. Lee: Dodecyl sulfate-induced fast faradic process in nickel cobalt oxide-reduced graphite oxide composite material and its application for asymmetric supercapacitor device. J. Mater. Chem. 22(43), 23114 (2012).
J. Liebscher, R. Mrówczyński, H.A. Scheidt, C. Filip, N.D. Hădade, R. Turcu, A. Bende, and S. Beck: Structure of polydopamine: A never-ending story?Langmuir 29(33), 10539 (2013).
M. Beidaghi and C.L. Wang: Micro-supercapacitors based on interdigital electrodes of reduced graphene oxide and carbon nanotube composites with ultrahigh power handling performance. Adv. Funct. Mater. 22(21), 4501 (2012).
P.L. Taberna, P. Simon, and J.F. Fauvarque: Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 150(3), A292 (2003).
M. Kaempgen, C.K. Chan, J. Ma, Y. Cui, and G. Gruner: Printable thin Film supercapacitors using single-walled carbon nanotubes. Nano Lett. 9(5), 1872 (2009).
Y. Liu, K. Ai, and L. Lu: Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 114(9), 5057 (2014).
N. Betz, A. Le Moël, E. Balanzat, J.M. Ramillon, J. Lamotte, J.P. Gallas, and G. Jaskierowicz: A FTIR study of PVDF irradiated by means of swift heavy ions. J. Polym. Sci., Part B: Polym. Phys. 32(8), 1493 (1994).
C.A. Pryde: IR studies of polyimides. I. Effects of chemical and physical changes during cure. J. Polym. Sci., Part A: Polym. Phys. 27(2), 711 (1989).
ACKNOWLEDGMENT
This research is supported by NRF Competitive Research Program NRF-CRP13-2014-02. Part of the work was carried out in the NTU-HUJ-BGU Nanomaterials for Energy and Water Management Program under the Campus for Research Excellence and Technological Enterprise (CREATE), that is supported by the National Research Foundation, Prime Minister’s Office, Singapore.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper has been selected as an Invited Feature Paper.
Supplementary Material
43578_2015_30233575_MOESM1_ESM.docx
Supplemental Material: A Polydopamine coated polyaniline single wall carbon nanotube composite material as a stable supercapacitor cathode in organic electrolyte (approximately 1.54 MB)
Rights and permissions
About this article
Cite this article
Wang, X., Lee, P.S. A polydopamine coated polyaniline single wall carbon nanotube composite material as a stable supercapacitor cathode in an organic electrolyte. Journal of Materials Research 30, 3575–3583 (2015). https://doi.org/10.1557/jmr.2015.342
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.342