Abstract
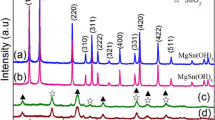
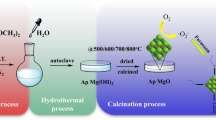
A phase transfer method was developed to prepare Mg(OH)2 nanosheets and a subsequent adsorption–calcination process was followed to obtain lamellar MgO nanostructures. The as-prepared MgO nanosheets also showed a superior adsorption property of Congo red. Transmission electron microscopy and x-ray diffractometer results indicated that the as-obtained Mg(OH)2 was plate-shaped with a hexagonal crystal structure where MgO possessed a lamellar structure with a cubic phase. The maximum adsorption capacities of Mg(OH)2 and MgO were reached up to 1820 and 2650 mg g−1, respectively. The high adsorption capacity might be related to the particle geometry and large surface area (87.97 m2 g−1 for Mg(OH)2 and 132.31 m2 g−1 for MgO). The adsorbents can be easily regenerated for five times without any significant loss in their adsorption property. The adsorption behaviors of the Mg(OH)2 and MgO adsorbents showed that the adsorption kinetics and isotherms were in good agreement with pseudo-second-order rate equation and Freundlich adsorption model.









Similar content being viewed by others
REFERENCES
G.E. Shter, H. Behar-Levy, V. Gelman, G.S. Grader, and D. Avnir: Organically doped metals—A new approach to metal catalysis: Enhanced Ag-catalyzed oxidation of methanol. Adv. Funct. Mater. 17 (6), 913 (2007).
R. Ahmad and R. Kumar: Adsorptive removal of Congo red dye from aqueous solution using bael shell carbon. Appl. Surf. Sci. 257 (5), 1628 (2010).
G. Zou, W. Chen, R. Liu, and Z. Xu: Morphology-tunable synthesis and characterizations of Mg(OH)2 films via a cathodic electrochemical process. Mater. Chem. Phys. 107 (1), 85 (2008).
T.I. Liakos and N.K. Lazaridis: Melanoidins removal from simulated and real wastewaters by coagulation and electro-flotation. Chem. Eng. J. 242, 269 (2014).
K. Turhan, I. Durukan, S.A. Ozturkcan, and Z. Turgut: Decolorization of textile basic dye in aqueous solution by ozone. Dyes Pigm. 92 (3), 897 (2012).
S. Yu, M. Liu, M. Ma, M. Qi, Z. Lü, and C. Gao: Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J. Membr. Sci. 350 (1–2), 83 (2010).
X. Gao, F. Xiao, C. Yang, J. Wang, and X. Su: Hydrothermal fabrication of W18O49 nanowire networks with superior performance for water treatment. J. Mater. Chem. A 1, 5831 (2013).
T. Hao, C. Yang, X. Rao, J. Wang, C. Niu, and X. Su: Facile additive-free synthesis of iron oxide nanoparticles for efficient adsorptive removal of Congo red and Cr (VI). Appl. Surf. Sci. 292, 174 (2013).
B. Wang, H. Wu, L. Yu, R. Xu, and T.T. Lim: Template-free formation of uniform urchin-like a-FeOOH hollow spheres with superior capability for water treatment. Adv. Mater. 24 (8), 1111 (2012).
B. Cheng, Y. Le, W. Cai, and J. Yu: Synthesis of hierarchical Ni(OH)2 and NiO nanosheets and their adsorption kinetics and isotherms to Congo red in water. J. Hazard. Mater. 185 (2–3), 889 (2011).
L. Kumari, W.Z. Li, C.H. Vannoy, R.M. Leblanc, and D.Z. Wang: Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO. Ceram. Int. 35 (8), 3355 (2009).
J. Hu, Z. Song, L. Chen, H. Yang, J. Li, and R. Richards: Adsorption properties of MgO (111) nanoplates for the dye pollutants from wastewater. J. Chem. Eng. Data 55 (9), 3742 (2010).
L. Todan, T. Dascalescu, S. Preda, C. Andronescu, C. Munteanu, D.C. Culita, A. Rusu, R. State, and M. Zaharescu: Porous nanosized oxide powders in the MgO-TiO2 binary system obtained by sol–gel method. Ceram. Int. 40 (10), 15693 (2014).
Z. Bouberka, K. Bentaleb, K.A. Benabbou, and U. Maschke: Adsorption of two dyes by Mg(OH)2: Procion blue HB and Remazol brilliant blue R. Springer Proc. Phys. 155, 463 (2014).
N.K. Nga, P.T. Hong, T.D. Lam, and T.Q. Huy: A facile synthesis of nanostructured magnesium oxide particles for enhanced adsorption performance in reactive blue 19 removal. J. Colloid Interface Sci. 398, 210 (2013).
C.Y. Cao, J. Qu, F. Wei, H. Liu, and W.G. Song: Superb adsorption capacity and mechanism of flowerlike magnesium oxide nanostructures for lead and cadmium ions. ACS Appl. Mater. Interfaces 4 (8), 4283 (2012).
Y. Li, C. Yang, J. Ge, C. Sun, J. Wang, and X. Su: A general microwave-assisted two-phase strategy for nanocrystals synthesis. J. Colloid Interface Sci. 407, 296 (2013).
W.F. Giauque and R.C. Archibald: The entropy of water from the third law of thermodynamics. The dissociation pressure and calorimetric heat of the reaction Mg(OH)2 = MgO + H2O. the heat capacities of Mg(OH)2 and MgO from 20 to 300°K. J. Am. Chem. Soc. 59 (3), 561 (1937).
S. Zhang, C. Deng, F.L. Liu, Q. Wu, M. Zhang, F.L. Meng, and H. Gao: Impacts of in situ carbon coating on the structural, morphological and electrochemical characteristics of Li2MnSiO4 prepared by a citric acid assisted sol–gel method. J. Electroanal. Chem. 689, 88 (2013).
N.C.S. Selvam, R.T. Kumar, L.J. Kennedy, and J.J. Vijaya: Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloys Compd. 509 (41), 9809 (2011).
M.A. Alavi and A. Morsali: Syntheses and characterization of Mg(OH)2 and MgO nanostructures by ultrasonic method. Ultrason. Sonochem. 17 (2), 441 (2010).
X-Y. Yu, T. Luo, Y. Jia, Y-X. Zhang, J-H. Liu, and X-J. Huang: Porous hierarchically micro-/nanostructured MgO: Morphology control and their excellent performance in As (III) and As (V) removal. J. Phys. Chem. C 115 (45), 22242 (2011).
M. Rezaei, M. Khajenoori, and B. Nematollahi: Synthesis of high surface area nanocrystalline MgO by pluronic P123 triblock copolymer surfactant. Powder Technol. 205 (1–3), 112 (2011).
L. Ai, H. Yue, and J. Jiang: Sacrificial template-directed synthesis of mesoporous manganese oxide architectures with superior performance for organic dye adsorption. Nanoscale 4 (17), 5401 (2012).
H. Dong, Z. Du, Y. Zhao, and D. Zhou: Preparation of surface modified nano-Mg(OH)2 via precipitation method. Powder Technol. 198 (3), 325 (2010).
A.M. Ruminski, K-J. Jeon, and J.J. Urban: Size-dependent CO2 capture in chemically synthesized magnesium oxide nanocrystals. J. Mater. Chem. 21 (31), 11486 (2011).
X. Li, W. Xiao, G. He, W. Zheng, N. Yu, and M. Tan: Pore size and surface area control of MgO nanostructures using a surfactant-templated hydrothermal process: High adsorption capability to azo dyes. Colloids Surf., A 408, 79 (2012).
P. Tian, X.Y. Han, G.L. Ning, H.X. Fang, J.W. Ye, W.T. Gong, and Y. Lin: Synthesis of porous hierarchical MgO and its superb adsorption properties. ACS Appl. Mater. Interfaces 5 (23), 12411 (2013).
J. Huang, W. Chen, W. Zhao, Y. Li, X. Li, and C. Chen: One-dimensional chainlike arrays of Fe3O4 hollow nanospheres synthesized by aging iron nanoparticles in aqueous solution. J. Phys. Chem. C 113 (28), 12067 (2009).
L. Wang, J. Li, Z. Wang, L. Zhao, and Q. Jiang: Low-temperature hydrothermal synthesis of α-Fe/Fe3O4 nanocomposite for fast Congo red removal. Dalton Trans. 42 (7), 2572 (2013).
T. Zhu, J.S. Chen, and X.W. Lou: Highly efficient removal of organic dyes from waste water using hierarchical NiO spheres with high surface area. J. Phys. Chem. C 116 (12), 6873 (2012).
Z. Zhang, Y. Shan, J. Wang, H. Ling, S. Zang, W. Gao, Z. Zhao, and H. Zhang: Investigation on the rapid degradation of Congo red catalyzed by activated carbon powder under microwave irradiation. J. Hazard. Mater. 147 (1), 325 (2007).
W. Cai, J. Yu, and M. Jaroniec: Template-free synthesis of hierarchical spindle-like γ-Al2O3 materials and their adsorption affinity towards organic and inorganic pollutants in water. J. Mater. Chem. 20 (22), 4587 (2010).
K.Y. Chong, C.H. Chia, S. Zakaria, and M.S. Sajab: Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. J. Environ. Chem. Eng. 2 (4), 2156 (2014).
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (51174174), Excellent Talents in Xinjiang Province (2013721015), and Postgraduate Education Innovation Project of Xinjiang Province (XJGRI2014016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributing Editor: Edson Roberto Leite
Rights and permissions
About this article
Cite this article
Liu, X., Niu, C., Zhen, X. et al. Novel approach for the synthesis of Mg(OH)2 nanosheets and lamellar MgO nanostructures and their ultra-high adsorption capacity for Congo red. Journal of Materials Research 30, 1639–1647 (2015). https://doi.org/10.1557/jmr.2015.113
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.113