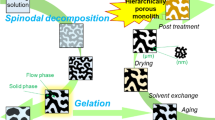
Abstract
In this article, monolithic porous silsesquioxane materials, which are derived by sol–gel from trialkoxysilanes with substituent groups such as trimethoxysilane (HTMS), methyltrimethoxysilane (MTMS), and vinyltrimethoxysilane (VTMS), are reviewed with a special emphasis on our recent works. Careful controls over fundamental synthetic parameters such as pH, amounts of water and solvent, and kind of solvent and additives play a crucial role in the formation of monolithic gels based on random polysiloxane networks. Crystalline/amorphous precipitation is otherwise observed when the formation of isolated species including polyhedral oligomeric silsesquioxanes dominates or if phase separation of the hydrophobic networks in aqueous media is not adequately controlled. In the successfully controlled system, pore size can be varied from a few tens of nanometers to a few tens of micrometers; porous materials such as transparent aerogels and hierarchically porous monoliths have been explored. In addition, unique properties derived from trialkoxysilanes such as reactivity of the pore surface and flexible mechanical properties are demonstrated. Possibilities in the silsesquioxane materials with controlled pore structures are discussed.











Similar content being viewed by others
Change history
01 January 2015
An Erratum to this paper has been published: https://doi.org/10.1557/jmr.2014.388
References
C. Sanchez and F. Ribot: Design of hybrid organic-inorganic materials synthesized via sol-gel chemistry. New J. Chem. 18, 53–63 (2006).
C. Sanchez, P. Belleville, M. Popall, and L. Nicole: Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 40, 696–753 (2011).
C. Sanchez, C. Boissiere, S. Cassaignon, C. Chaneac, O. Durupthy, M. Faustini, D. Grosso, C. Laberty-Robert, L. Nicole, D. Portehault, F. Ribot, L. Rozes, and C. Sassoye: Molecular engineering of functional inorganic and hybrid materials. Chem. Mater. 26, 221–238 (2014).
H. Schmidt and H. Wolter: Organically modified ceramics and their applications. J. Non-Cryst. Solids 121, 428–435 (1990).
B. Novak: Hybrid nanocomposite materials–Between inorganic glasses and organic polymers. Adv. Mater. 5, 422–433 (1993).
R.J.P. Corriu and D. Leclercq: Recent developments of molecular chemistry of sol-gel processing. Angew. Chem., Int. Ed. Engl. 35, 1420–1436 (1996).
D. Avnir: Organic chemistry within ceramic matrices: Doped sol-gel materials. Acc. Chem. Res. 28, 328–334 (1995).
T. Ogoshi and Y. Chujo: Organic-inorganic polymer hybrids prepared by the sol-gel method. Compos. Interfaces 11, 539–566 (2005).
D. Avnir, T. Coradin, O. Lev, and J. Livage: Recent bio-applications of sol-gel materials. J. Mater. Chem. 16, 1013–1030 (2006).
B. Dunn and J.I. Zink: Molecules in glass: Probes, ordered assemblies, and functional materials. Acc. Chem. Res. 40, 747–755 (2007).
K. Matsui: Entrapment of organic molecules. In Handbook of Sol-Gel Science and Technology: Processing Characterization and Applications, S. Sakka ed.; Kluwer Academic Publishers: Dordrecht, Vol. I, 2004; pp. 459–484.
P. Colombo, G. Mera, R. Riedel, and G.D. Sorarù: Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 93, 1805–1837 (2010).
C.G. Pantano, A.K. Singh, and H. Zhang: Silicon oxycarbide glasses. J. Sol-Gel Sci. Technol. 14, 7–25 (1999).
K. Kamiya: Oxynitride glasses and nitrides. In Handbook of Sol-Gel Science and Technology: Processing Characterization and Applications, S. Sakka ed.; Kluwer Academic Publishers: Dordrecht, Vol. I, 2004; pp. 171–183.
K. Kamiya: Oxycarbide glasses and carbides. In Handbook of Sol-gel Science and Technology: Processing Characterization and Applications, S. Sakka, ed.; Kluwer Academic Publishers: Dordrecht, Vol. I, 2004; pp. 185–201.
A.R. Studart, U.T. Gonzenbach, E. Tervoort, and L.J. Gauckler: Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 89, 1771–1789 (2006).
P. Colombo:Engineering porosity in polymer-derived ceramics. J. Eur. Ceram. Soc. 28, 1389–1395 (2008).
K. Kanamori and K. Nakanishi: Controlled pore formation in organotrialkoxysilanes-derived hybrids: From aerogels to hierarchically porous monoliths. Chem. Soc. Rev. 40, 754–770 (2011).
R.H. Baney, M. Itoh, A. Sakakibara, and T. Suzuki: Silsesquioxanes. Chem. Rev. 95, 1409–1430 (1995).
M.A. Brook: Silicon in Organic, Organometallic, and Polymer Chemistry (John Wiley & Sons, New York, 2000).
W. Volksen, R.D. Miller, and G. Dubois: Low dielectric constant materials. Chem. Rev. 110, 56–110 (2010).
B.A. Kamino and T.P. Bender: The use of siloxanes, silsesquioxanes, and silicones in organic semiconducting materials. Chem. Soc. Rev. 42, 5119–5130 (2013).
K. Tanaka, F. Ishiguro, and Y. Chujo: POSS ionic liquid. J. Am. Chem. Soc. 132, 17649–17651 (2010).
K. Tanaka and Y. Chujo: Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 22, 1733–1746 (2012).
P.R. Chinnam and S.L. Wunder: Polyoctahedral silsesquioxane-nanoparticle electrolytes for lithium batteries: POSS-lithium salts and POSS-PEGs. Chem. Mater. 23, 5111–5121 (2011).
Z. Chu and S. Seeger: Superamphiphobic surfaces. Chem. Soc. Rev. 43, 2784–2798 (2014).
H.L. Castricum, G.G. Paradis, M.C. Mittelmeijer-Hazeleger, R. Kreiter, J.F. Vente, and E. ten Elshof: Tailoring the separation behavior of hybrid organosilica membranes by adjusting the structure of the organic bridging group. Adv. Funct. Mater. 21, 2319–2329 (2011).
R. Xu, J. Wang, M. Kanezashi, T. Yoshioka, and T. Tsuru: Development of robust organosilica membranes for reverse osmosis. Langmuir 27, 13996–13999 (2011).
Y.T. Chua, C.X.C. Lin, F. Kleitz, X.S. Zhao, and S. Smart: Nanoporous organosilica membrane for water desalination. Chem. Commun. 49, 4534–4536 (2013).
S-W. Kuo and F-C. Chang: POSS related polymer nanocomposites. Prog. Polym. Sci. 36, 1649–1696 (2011).
B. Lebeau and P. Innocenzi: Hybrid materials for optics and photonics. Chem. Soc. Rev. 40, 886–906 (2011).
S. Fujita and S. Inagaki: Self-organization of organosilica solids with molecular-scale and mesoscale periodicities. Chem. Mater. 20, 891–908 (2008).
B. Lebeau, F. Gaslain, C. Fernandez-Martin, and F. Babonneau: Organically modified ordered mesoporous siliceous solids. In Ordered Porous Solids: Recent Advances and Prospects, V. Valtchev, S. Mintova, and M. Tsapatsis eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 283–308.
N. Mizoshita, T. Tani, and S. Inagaki: Syntheses, properties and applications of periodic mesoporous organosilicas prepared from bridged organosilane precursors. Chem. Soc. Rev. 40, 789–800 (2011).
P. Van Der Voort, D. Esquivel, E. De Canck, F. Goethals, I. Van Driessche, and F.J. Romero-Salguero: Periodic mesoporous organosilicas: From simple to complex bridges; a comprehensive overview of functions, morphologies and applications. Chem. Soc. Rev. 42, 3913–3955 (2013).
K. Nakanishi: Pore structure control of silica gels based on phase separation. J. Porous Mater. 4, 67–112 (1997).
K. Nakanishi and N. Tanaka: Sol-gel with phase separation. Hierarchically porous materials optimized for high-performance liquid chromatography separations. Acc. Chem. Res. 40, 863–873 (2007).
K. Nakanishi: Synthesis concepts and preparation of silica monoliths. In Monolithic Silicas in Separation Science, K.K. Unger, N. Tanaka, and E. Machtejevas eds.; Wiley-VCH: Weinheim, 2011; pp. 11–33.
G. Hasegawa, K. Kanamori, K. Nakanishi, and T. Hanada: Fabrication of macroporous silicon carbide ceramics by intramolecular carbothermal reduction of phenyl-bridged polysilsesquioxane. J. Mater. Chem. 19, 7716–7720 (2009).
G. Hasegawa, K. Kanamori, K. Nakanishi, and T. Hanada: Hierarchically porous carbon monoliths with high surface area from bridged polysilsesquioxanes without thermal activation process. Chem. Commun. 46, 8037–8039 (2010).
G. Hasegawa, K. Kanamori, K. Nakanishi, and T. Hanada: A new route to monolithic macroporous SiC/C composites from biphenylene-bridged polysilsesquioxane gels. Chem. Mater. 22, 2541–2547 (2010).
K.J. Shea and D.A. Loy: Bridged polysilsesquioxanes. Molecular-engineered hybrid organic-inorganic materials. Chem. Mater. 13, 3306–3319 (2001).
D.A. Loy, B.M. Baugher, C.R. Baugher, D.A. Schneider, and K. Rahimian: Substituent effects on the sol-gel chemistry of organotrialkoxysilanes. Chem. Mater. 12, 3624–3632 (2000).
C.J. Brinker and G.W. Scherer: Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press, San Diego, 1990), Chapter 3.
S. Che, Z. Liu, T. Osuna, K. Sakamoto, O. Terasaki, and T. Tatsumi: Synthesis and characterization of chiral mesoporous silica. Nature 429, 281–284 (2004).
A. Shimojima and K. Kuroda: Designed synthesis of nanostructured siloxane–organic hybrids from amphiphilic silicon-based precursors. Chem. Rec. 6, 53–63 (2006).
C.J. Brinker and G.W. Scherer: Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. (Academic Press, San Diego, CA, 1990), Chapter 5.
D.B. Cordes, P.D. Lickiss, and F. Rataboul: Recent development in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 110, 2081–2173 (2010).
L.V. Ng, P. Thompson, J. Sanchez, C.W. Macosko, and A.V. McCormick: Formation of cagelike intermediates from nonrandom cyclization during acid-catalyzed sol-gel polymerization of tetraethyl orthosilicate. Macromolecules 28, 6471–6476 (1995).
M.J. Mora-Fonz, C.R.A. Catlow, and D.W. Lewis: Oligomerization and cyclization processes in the nucleation of microporous silicas. Angew. Chem., Int. Ed. 44, 3082–3086 (2005).
C. Zhang, F. Babonneau, C. Bonhomme, R.M. Laine, C.L. Soles, H.A. Hristov, and A.F. Yee: Highly porous polyhedral silsesquioxane polymers. Synthesis and characterization. J. Am. Chem. Soc. 120, 8380–8391 (1998).
H. Guo, M.A.B. Meador, L. McCorkle, D.J. Quade, J. Guo, B. Hamilton, M. Cakmak, and G. Sprowl: Polyimide aerogels cross-linked through amine functionalized polyoligomeric silsesquioxane. ACS Appl. Mater. Interfaces 3, 546–552 (2011).
H. Lin, J. Ou, Z. Zhang, J. Dong, and H. Zou: Ring-opening polymerization reaction of polyhedral oligomeric silsesquioxanes (POSSs) for preparation of well-controlled 3D skeletal hybrid monoliths. Chem. Commun. 49, 231–233 (2013).
H. Dong, M. Lee, R.D. Thomas, Z. Zhang, R.F. Reidy, and D.W. Mueller: Methyltrimethoxysilane sol-gel polymerization in acidic ethanol solutions studied by 29Si NMR spectroscopy. J. Sol-Gel Sci. Technol. 28, 5–14 (2003).
H. Dong, Z. Zhang, M-H. Lee, D.W. Mueller, and R.F. Reidy: Sol-gel polycondensation of methyltrimethoxysilane in ethanol studied by 29Si NMR spectroscopy using a two-step acid/base procedure. J. Sol-Gel Sci. Technol. 41, 11–17 (2007).
K. Kanamori, Y. Kodera, G. Hayase, K. Nakanishi, and T. Hanada: Transition from transparent aerogels to hierarchically porous monoliths in polymethylsilsesquioxane sol-gel system. J. Colloid Interface Sci. 357, 336–344 (2011).
O. Riant, N. Mostefaï, and J. Courmarcel: Recent advances in the asymmetric hydrosilylation of ketones, imines and electrophilic double bonds. Synthesis 18, 2943–2958 (2004).
R.H. Morris: Asymmetric hydrogenation, transfer hydrogenation and hydrosilylation of ketones catalyzed by iron complexes. Chem. Soc. Rev. 38, 2282–2291 (2009).
D. Addis, S. Das, K. Junge, and M. Beller: Selective reduction of carboxylic acid derivatives by catalytic hydrosilylation. Angew. Chem., Int. Ed. 50, 6004–6011 (2011).
N. Moitra, K. Kanamori, T. Shimada, K. Takeda, Y.H. Ikuhara, X. Gao, and K. Nakanishi: Synthesis of hierarchically porous hydrogen silsesquioxane monoliths and embedding of metal nanoparticles by on-site reduction. Adv. Funct. Mater. 23, 2714–2722 (2013).
Z. Xie, E.J. Henderson, Ö. Dag, W. Wang, J.E. Lofgreen, C. Kübel, T. Scherer, P.M. Brodersen, Z-Z. Gu, and G.A. Ozin: Periodic mesoporous hydridosilica–Synthesis of an “impossible” material and its thermal transformation into brightly photoluminescent periodic mesoporous nanocrystal silicon-silica composite. J. Am. Chem. Soc. 133, 5094–5102 (2011).
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, and G.D. Stucky: Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998).
G.D. Sorarù, G. D’Andrea, R. Campostrini, F. Babonneau, and G. Mariotto: Structural characterization and high-temperature behavior of silicon oxycarbide glasses prepared from sol-gel precursors containing Si-H bonds. J. Am. Ceram. Soc. 78, 379–387 (1995).
H-J. Kleebe and Y.D. Blum: SiOC ceramic with high excess free carbon. J. Eur. Ceram. Soc. 28, 1037–1042 (2008).
C.M. Hessel, E.J. Henderson, and J.G.C. Veinot: Hydrogen silsesquioxane: A molecular precursor for nanocrystalline Si-SiO2 composites and freestanding hydride-surface-terminated silicon nanoparticles. Chem. Mater. 18, 6139–6146 (2006).
Ö. Dag, E.J. Henderson, W. Wang, J.E. Lofgreen, S. Petrov, P.M. Brodersen, and G.A. Ozin: Spatially confined redox chemistry in periodic mesoporous hydridosilica-nanosilver grown in reducing nanopores. J. Am. Chem. Soc. 133, 17454–17462 (2011).
N. Moitra, K. Kanamori, Y.H. Ikuhara, X. Gao, Z. Yang, G. Hasegawa, K. Takeda, T. Shimada, and K. Nakanishi: Reduction on reactive pore surface as a versatile approach to monolith-supported metal alloy nanoparticles and its catalytic applications. J. Mater. Chem. A 2, 12535–12544 (2014).
N. Moitra, A. Matsushima, T. Kamei, K. Kanamori, Y.H. Ikuhara, X. Gao, K. Takeda, Y. Zhu, K. Nakanishi, and T. Shimada: A new hierarchically porous Pd@HSQ monolithic catalyst for Mizoroki-Heck cross-coupling reaction. New J. Chem. 38, 1144–1149 (2014).
N. Moitra, T. Kamei, K. Kanamori, K. Nakanishi, K. Takeda, and T. Shimada: Recyclable functionalization of silica with alcohols via dehydrogenative addition on hydrogen silsesquioxane. Langmuir 29, 12243–12253 (2013).
T. Shimada, K. Aoki, Y. Shinoda, T. Nakamura, N. Tokunaga, S. Inagaki, and T. Hayashi: Functionalization on silica gel with allylsilanes. A new method of covalent attachment of organic functional groups on silica gel. J. Am. Chem. Soc. 125, 4688–4689 (2003).
J-W. Park and C-H. Jun: Transition-metal-catalyzed immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions. J. Am. Chem. Soc. 132, 7268–7269 (2010).
H. Dong, M.A. Brook, and J.D. Brennan: A new route to monolithic methylsilsesquioxanes: Gelation behavior of methyltrimethoxysilane and morphology of resulting methylsilsesquioxanes under one-step and two-step processing. Chem. Mater. 17, 2807–2816 (2005).
K. Kanamori, H. Yonezawa, K. Nakanishi, K. Hirao, and H. Jinnai: Structural formation of hybrid siloxane-based polymer monolith in confined spaces. J. Sep. Sci. 27, 874–886 (2004).
K. Nakanishi and K. Kanamori: Organic-inorganic hybrid poly(silsesquioxane) monoliths with controlled macro- and mesopores. J. Mater. Chem. 15, 3776–3786 (2005).
K. Kanamori, K. Nakanishi, and T. Hanada: Thick silica gel coatings on methylsilsesquioxane monoliths using anisotropic phase separation. J. Sep. Sci. 29, 2463–2470 (2006).
K. Kanamori, M. Aizawa, K. Nakanishi, and T. Hanada: New transparent methylsilsesquioxane aerogels and xerogels with improved mechanical properties. Adv. Mater. 19, 1589–1593 (2007).
K. Kanamori, M. Aizawa, K. Nakanishi, and T. Hanada: Elastic organic-inorganic hybrid aerogels and xerogels. J. Sol-Gel Sci. Technol. 48, 172–181 (2008).
K. Kanamori, K. Nakanishi, and T. Hanada: J. Ceram. Soc. Jpn. 117, 1333–1338 (2009).
G. Hayase, K. Kanamori, and K. Nakanishi: Structure and properties of polymethylsilsesquioxane aerogels synthesized with surfactant n -hexadecyltrimethylammonium chloride. Microporous Mesoporous Mater. 158, 247–252 (2012).
M. Kurahashi, K. Kanamori, K. Takeda, H. Kaji, and K. Nakanishi: Role of block copolymer surfactant on the pore formation in methylsilsesquioxane aerogel systems. RSC Adv. 2, 7166–7173 (2012).
N. Hüsing and U. Schubert: Aerogels-airy materials: Chemistry, structure, and properties. Angew. Chem., Int. Ed. 37, 22–45 (1998).
A.C. Pierre and G.M. Pajonk: Chemistry of aerogels and their applications. Chem. Rev. 102, 4243–4265 (2002).
M. Koebel, A. Rigacci, and P. Achard: Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 63, 315–339 (2012).
H. Itoh, T. Tabata, M. Kokitsu, N. Okazaki, Y. Imizu, and A. Tada: Preparation of SiO2-Al2O3 gels from tetraethoxysilane and aluminum chloride. J. Ceram. Soc. Jpn. 101, 1081–1083 (1993).
A.E. Gash, T.M. Tillotson, J.H. Satcher, Jr., J.F. Poco, L.W. Hrubesh, and R.L. Simpson: Use of epoxides in the sol-gel synthesis of porous iron(III) oxide monoliths from Fe(III) salts. Chem. Mater. 13, 999–1007 (2001).
X. Guo, W. Li, H. Yang, K. Kanamori, Y. Zhu, and K. Nakanishi: Gelation behavior and phase separation of macroporous methylsilsesquioxane monoliths prepared by in situ two-step processing. J. Sol-Gel Sci. Technol. 67, 406–413 (2013).
X. Guo, H. Yu, H. Yang, K. Kanamori, Y. Zhu, and K. Nakanishi: Pore structure control of macroporous methylsilsesquioxane monoliths prepared by in situ two-step processing. J. Porous Mater. 20, 1477–1483 (2013).
J. Cai, S. Liu, J. Feng, S. Kimura, M. Wada, S. Kuga, and L. Zhang: Cellulose-silica nanocomposite aerogels by in situ formation of silica in cellulose gel. Angew. Chem., Int. Ed. 51, 2076–2079 (2012).
M.A. Worsley, S.O. Kucheyev, J.D. Kuntz, T.Y. Olson, T.Y.-J. Han, A.V. Hamza, J.H. Satcher, Jr., and T.F. Baumann: Carbon scaffolds for stiff and highly conductive monolithic oxide-carbon nanotube composites. Chem. Mater. 23, 3054–3061 (2011).
D.J. Boday, B. Muriithi, R.J. Stover, and D.A. Loy: Polyaniline nanofiber-silica composite aerogels. J. Non-Cryst. Solids 358, 1575–1580 (2012).
G. Hayase, K. Kanamori, K. Abe, H. Yano, A. Maeno, H. Kaji, and K. Nakanishi: Polymethylsilsesquioxane-cellulose nanofiber biocomposite aerogels with high thermal insulation, bendability and superhydrophobicity. ACS Appl. Mater. Interfaces (published online. DOI: https://doi.org/10.1021/am501822y).
G. Hayase, K. Kanamori, and K. Nakanishi: New flexible aerogels and xerogels derived from methyltrimethoxysilane/dimethyldimethoxysilane co-precursors. J. Mater. Chem. 21, 17077–17079 (2011).
G. Hayase, K. Kanamori, G. Hasegawa, A. Maeno, H. Kaji, and K. Nakanishi: A superamphiphobic macroporous silicone monolith with marshmallow-like flexibility. Angew. Chem., Int. Ed. 52, 1986–1989 (2013).
G. Hayase, K. Kanamori, M. Fukuchi, H. Kaji, and K. Nakanishi: Facile synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water. Angew. Chem., Int. Ed. 52, 1986–1989 (2013).
J. Wen and G.L. Wilkes: Organic/inorganic hybrid network materials by the sol-gel approach. Chem. Mater. 8, 1667–1681 (1996).
B.M. Novak, D. Auerbach, and C. Verrier: Low-density, mutually interpenetrating organic-inorganic composite materials via supercritical drying techniques. Chem. Mater. 4, 282–286 (1994).
S.J. Kramer, F. Rubio-Alonso, and J.D. Mackenzie: Organically modified silicate aerogels, “aeromosils”. Mater. Res. Soc. Symp. Proc. 435, 295–300 (1996).
J.D. Mackenzie and E.P. Bescher: Mechanical properties of organic-inorganic hybrids. In Handbook of Sol-Gel Science and Technology: Processing Characterization and Applications, S. Sakka ed.; Kluwer Academic Publishers: Dordrecht, 2004, Vol. II; pp. 313–326.
H. Frenkel-Mullerad and D. Avnir: The chemical reactivity of sol-gel materials: Hydrobromination of ormosils. Chem. Mater. 12, 3754–3759 (2000).
A. Itagaki, K. Nakanishi, and K. Hirao: Phase separation in sol-gel system containing mixture of 3- and 4-functional alkoxysilanes. J. Sol-Gel Sci. Technol. 26, 153–156 (2003).
A. Shimojima and K. Kuroda: Designed synthesis of nanostructured siloxane-organic hybrids from amphiphilic silicon-based precursors. Chem. Rec. 6, 53–63 (2006).
K. Kuroda, A. Shimojima, K. Kawahara, R. Wakabayashi, Y. Tamura, Y. Asakura, and M. Kitahara: Utilization of alkoxysilyl groups for the creation of structurally controlled siloxane-based nanomaterials. Chem. Mater. 26, 211–220 (2014).
J.N. Hay, D. Porter, and H.M. Raval: A versatile route to organically-modified silicas and porous silicas via the non-hydrolytic sol-gel process. J. Mater. Chem. 10, 1811–1818 (2000).
P.H. Mutin and A. Vioux: Nonhydrolytic processing of oxide-based materials: Simple routes to control homogeneity, morphology, and nanostructure. Chem. Mater. 21, 582–596 (2009).
Y. Liu, M. Wang, Z. Li, H. Liu, P. He, and J. Li: Preparation of porous aminopropylsilsesquioxane by a nonhydrolytic sol-gel method in ionic liquid solvent. Langmuir 21, 1618–1622 (2005).
A. Arkhireeva, J.N. Hay, and M. Manzano: Preparation of silsesquioxane particles via a nonhydrolytic sol-gel route. Chem. Mater. 17, 875–880 (2005).
A. González-Campo, E.J. Juárez-Pérez, C. Viñas, B. Boury, R. Sillanpää, R. Kivekäs, and R. Núñez: Carboranyl substituted siloxanes and octasilsesquioxanes: Synthesis, characterization, and reactivity. Macromolecules 41, 8458–8466 (2008).
D.J. Boday, S. Tolbert, M.W. Keller, Z. Li, J.T. Wertz, B. Muriithi, and D.A. Loy: Non-hydrolytic formation of silica and polysilsesquioxane particles from alkoxysilane monomers with formic acid in toluene/tetrahydrofuran solutions. J. Nanopart. Res. 16, 2313 (2014).
ACKNOWLEDGMENTS
The author is grateful for all co-workers who have contributed to the works reviewed in the present article (most of the names appear in the reference list). Financial supports such as Grants-in-Aid for Scientific Research (administrated by Japan Society for the Promotion of Science and Ministry of Education, Culture, Sports, Science and Technology, Japan) and Advanced Low Carbon Technology Research and Development Program (ALCA, by Japan Science and Technology Agency) are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper has been selected as an Invited Feature Paper.
Rights and permissions
About this article
Cite this article
Kanamori, K. Monolithic silsesquioxane materials with well-defined pore structure. Journal of Materials Research 29, 2773–2786 (2014). https://doi.org/10.1557/jmr.2014.332
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2014.332