Abstract
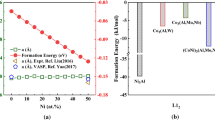
The structural properties, the formation enthalpies, and the mechanical properties of Co–Al compounds (CoAl, CoAl3, Co3Al, Co2Al5, Co2Al9, and Co4Al13) are studied by using Chen’s lattice inversion embedded-atom method. The potential is transferable and therefore does well for studying different Co–Al compounds. The calculated lattice parameters and cohesive energies are consistent with the experimental and theoretical results. The formation enthalpies of all the Co–Al compounds are negative; therefore, the chemical bonding between Co and Al atoms increases the stability of compounds. According to elastic constant restrictions, all the six Co–Al compounds are mechanically stable. CoAl alloy with the larger moduli and lower Poisson’s ratio is the hard or brittle phase. Moreover, CoAl3, Co3Al, Co2Al5, and Co2Al9 alloys are considered to be ductile materials, which have lower ratio of shear modulus to bulk modulus.






Similar content being viewed by others
References
W. Steurer: Twenty years of structure research on quasicrystals. Part I. Pentagonal, octagonal, decagonal and dodecagonal quasicrystals. Z. Kristallogr. 219, 391 (2004).
F. Fleischer, T. Weber, D.Y. Jung, and W. Steurer: s-Al13Co4, a new quasicrystal approximant. J. Alloys Compd. 500, 153 (2010).
M. Heggen, D.W. Deng, and M. Feuerbacher: Plastic deformation properties of the orthorhombic complex metallic alloy phase Al13Co4. Intermetallics 15, 1425 (2007).
M. Mihalkovic and M. Widom: First-principles calculations of cohesive energies in the Al-Co binary alloy system. Phys. Rev. B 75, 014207 (2007).
M. Mizuno, H. Araki, and Y. Shirai: Energetics and structural relaxation of constitutional defects in CoAl and CoTi from first principles. Phys. Rev. B 68, 144103 (2003).
C. Bergman, C. Girardeaux, C. Perrin-Pellegrino, P. Gas, D. Chatain, J.M. Dubois, and N. Rivier: Wetting of decagonal Al13Co4 and cubic AlCo thin films by liquid Pb. Philos. Mag. 86, 849 (2006).
M. Heggen, L. Houben, and M. Feuerbacher: Metadislocations in the structurally complex orthorhombic alloy Al13Co4. Philos. Mag. 88, 2333 (2008).
J. Dolinšek, M. Komelj, P. Jeglic, S. Vrtnik, D. Stanic, P. Popcevic, J. Ivkov, A. Smontara, Z. Jaglicic, and P. Gille: Anisotropic magnetic and transport properties of orthorhombic Al13Co4. Phys. Rev. B 79, 184201 (2009).
H-Z. Luo, Z-Y. Zhu, L. Ma, S-F. Xu, G-H. Wu, H-Y. Liu, J-P. Qu, Y-X. Li, X-X. Zhu, C-B. Jiang, and H-B. Xu: Effect of Cr on the electronic structure of Co3Al intermetallic compound: A first-principles study. J. Magn. Magn. Mater. 320, 1345 (2008).
V.K. Portnoi, K.V. Tretyakov, and V.I. Fadeeva: Structural transformations during the mechanochemical synthesis and heating of Co-Al Alloys. Inorg. Mater. 40, 937 (2004).
G.V. Golubkova, O.I. Lomovsky, Y.S. Kwon, A.A. Vlasov, and A.L. Chuvilin: Formation of nanocrystalline structures in a Co-Al system by mechanical alloying and leaching. J. Alloys Compd. 351, 101 (2003).
A. Ormeci and Y. Grin: Chemical bonding in Al5Co2: The electron localizability-electron density approach. Isr. J. Chem. 51, 1349 (2011).
V.K. Portnoi, K.V. Tretyakov, V.I. Fadeeva, and J. Latuch: Effects of liquid quenching and subsequent heating on the structure of Co-Al Alloys. Inorg. Mater. 41, 350 (2005).
C. Vailhé and D. Farkas: Shear faults and dislocation core structures in B2 CoAl. J. Mater. Res. 12, 2559 (1997).
B-W. Zhang, W-Y. Hu, and X-L. Su: Theory of Embedded Atom Method and its Application to Materials Science-atomic Scale Materials Design Theory (Hunan University Press, Hunan, China, 2003), p. 397.
W-P. Dong, H-K. Kim, W-S. Ko, B-M. Lee, and B-J. Lee: Atomistic modeling of pure Co and Co-Al system. Calphad 38, 7 (2012).
C-H. Zhang, J-J. Han, S. Huang, and J. Shen: Chen’s lattice inversion embedded-atom method for FCC metal. Adv. Mater. Res. 320, 415 (2011).
C-H. Zhang, S. Huang, J. Shen, and N-X. Chen: Chen’s lattice inversion embedded-atom method for Ni-Al alloy. Chin. Phys. B 21, 113401 (2012).
M.S. Daw and M.I. Baskes: Semiempirical, quantum mechanical calculation of hydrogen embrittlement in metals. Phys. Rev. Lett. 50, 1285 (1983).
M.S. Daw and M.I. Baskes: Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 29, 6443 (1984).
P.M. Morse: Diatomic molecules according to the wave mechanics II. Vibrational levels. Phys. Rev. 34, 57 (1929).
A. Banerjea and J.R. Smith: Origins of the universal binding-energy relation. Phys. Rev: B, Condens. Matter 37, 6632 (1988).
J.K. Norskov and N.D. Lang: Effective-medium theory of chemical binding: Application to chemisorptions. Phys. Rev. B 21, 2131 (1980).
J.H. Rose, J.R. Smith, F. Guinea, and J. Ferrante: Universal features of the equation of state of metals. Phys. Rev. B 29, 2963 (1984).
N-X. Chen: Modified mobius inverse formula and its applications in physics. Phys. Rev. Lett. 64, 1193 (1990).
N-X. Chen, Z-D. Chen, and Y-C. Wei: Multidimensional inverse lattice problem and a uniformly sampled arithmetic fourier transform. Phys. Rev. E 55, R5 (1998).
S. Huang, C-H. Zhang, J. Sun, Y-P. Li, Z-F. Zhang, and J. Shen: Formation and migration mechanism of the vacancy in three typical structures metal. Appl. Phys. 2, 50 (2012).
M.D. Segall, P.J.D. Lindan, M.J. Probert, C.J. Pickard, P.J. Hasnip, S.J. Clark, and M.C. Payne: First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys: Condens. Matter 14, 2717 (2002).
P. Villars and L. Calvert: Pearson’s Handbook Desk Edition: Crystallographic Data for Intermetallic Phases, 2nd ed. (ASM International, Materials Park, OH, 1997), p. 135.
M.J. Cooper: An investigation of the ordering of the phases CoAl and NiAl. Philos. Mag. 8, 805 (1963).
R. Hultgren, P. Desai, D. Hawkins, N. Gleiser, and K. Kelly: Selected Values of Thermodynamic Properties of Binary Alloys (ASM International, Materials Park, OH, 1973), p. 214.
H.П. ЛЯКИШeB: Handbook of Binary Alloy Phase Diagrams (Beijing Chemical Industry Press, Beijing, China, 2009), p. 52.
O.L. Anderson: A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 24, 909 (1963).
J.D. Gale and A.L. Rohl: The general utility lattice program. Mol. Simul. 29, 291 (2003).
M.J. Mehl, J.E. Osburn, D.A. Papaconstantopoulos, and B.M. Klein: Structural properties of ordered high-melting-temperature intermetallic alloys from first-principles total-energy calculations. Phys. Rev. B 41, 10311 (1990).
M.F. Grosso, H.O. Mosca, and G. Bozzolo: Thermal and physical properties of B2 Al-Ir-X (X = Ni, Ru, Pd, Co, Fe) alloys. Intermetallics 18, 945 (2010).
H. Ohtani, M. Yamano, and M. Hasebe: Thermodynamic analysis of the Co-Al-C and Ni-Al-C systems by incorporating ab initio energetic calculations into the CALPHAD approach. Calphad 28, 177 (2004).
M. Widom and J.A. Moriarty: First-principles interatomic potentials for transition-metal aluminides. II. Application to Al-Co and Al-Ni phase diagrams. Phys. Rev. B 58, 8967 (1998).
S.R. Broderick, H. Aourag, and K. Rajan: Data mining density of states spectra for crystal structure classification: An inverse problem approach. Stat. Anal. Data Min. 1, 353 (2009).
G. Trambly de Laissardiere, D. Nguyen Manh, L. Magaud, J.P. Julien, F. Cyrot-Lackmann, and D. Mayou: Electronic structure and hybridization effects in hume-rothery alloys containing transition elements. Phys. Rev. B 52, 7920 (1995).
J.B. Newkirk, P.J. Balck, and A. Damjanovic: The refinement of the Co2Al5 structures. Acta Crystallogr. 14, 532 (1961).
J.A. Moriarty and M. Widom: First-principles interatomic potentials for transition-metal aluminides: Theory and trends across the 3d series. Phys. Rev. B 56, 7905 (1997).
X-L. Ma and K-H. Kuo: Decagonal quasicrystal and related crystalline phases in slowly solidified Al-Co alloys. Metall. Mater. Trans. A 23, 1121 (1992).
R.C. Hudd and W.H. Taylor: The structure of Co4Al13. Acta Crystallogr. 15, 441 (1962).
J. Grin, U. Burkhardt, M. Ellner, and K. Peters: Crystal structure of orthorhombic Co4Al13. J. Alloys Compd. 206, 243 (1994).
J.F. Nye: Physical Properties of Crystals (Oxford University Press, Oxford, England, 1985), p. 85.
O. Beckstein, J.E. Klepeis, G.L.W. Hart, and O. Pankratov: First-principles elastic constants and electronic structure of a-Pt2Si and PtSi. Phys. Rev. B 63, 134112 (2001).
T. Tsuchiya, T. Yamanaka, and M. Matsui: Molecular dynamics study of pressure-induced transformation of quartz-type GeO2. Phys. Chem. Miner. 27, 149 (2000).
R.L. Fleischer: Substitutional solutes in AlRu-I. Effects of solute on moduli, lattice parameters and vacancy production. Acta Metall. Mater. 41, 863 (1993).
J. Haines, J.M. Leger, and G. Bocquillon: Synthesis and design of superhard materials. Annu. Rev. Mater. Res. 31, 1 (2001).
S.F. Pugh: XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 45, 823 (1954).
J. Schroers and W.L. Johnson: Ductile bulk metallic glass. Phys. Rev. Lett. 93, 255506 (2004).
K. Gschneidner, A. Russell, A. Pecharsky, J. Morris, Z. Zhang, T. Lograsso, D. Hsu, C.H. Lo, Y. Ye, A. Slager, and D. Kesse: A family of ductile intermetallic compounds. Nat. Mater. 2, 587 (2003).
Acknowledgment
This work was supported in part by the National Basic Research Program of China under Grant No. 2011CB606400.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, CH., Huang, S., Shen, J. et al. Atomistic modeling of Co–Al compounds. Journal of Materials Research 28, 2720–2727 (2013). https://doi.org/10.1557/jmr.2013.255
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2013.255