Abstract
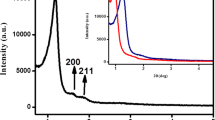
Confining light metal hydrides in micro- or mesoporous scaffolds is considered to be a promising way to overcome the existing challenges for these materials, e.g. their application in hydrogen storage. Different techniques exist which allow us to homogeneously fill pores of a host matrix with the respective hydride, thus yielding well defined composite materials. For this report, the ordered mesoporous carbon CMK-3 was taken as a support for LiAlH4 realized by a solution impregnation method to improve the hydrogen desorption behavior of LiAlH4 by nanoconfinement effects. It is shown that upon heating, LiAlH4 is unusually oxidized by coordinated tetrahydrofuran solvent molecules. The important result of the herein described work is the finding of a final composite containing nanoscale aluminum oxide inside the pores of the CMK-3 carbon host instead of a metal or alloy. This newly observed unusual oxidation behavior has major implications when applying these compounds for the targeted synthesis of homogeneous metal–carbon composite materials.








Similar content being viewed by others
References
A. Andreasen, T. Vegge, and A.S. Pedersen: Dehydrogenation kinetics of as-received and ball-milled LiAlH4. J. Solid State Chem. 178, 3672 (2005).
J. Gao, P. Adelhelm, M.H.W. Verkuijlen, C. Rongeat, M. Herrich, P.J.M. Van Bentum, O. Gutfleisch, A.P.M. Kentgens, K.P. De Jong, and P.E. De Jongh: Confinement of NaAlH4 in nanoporous carbon: Impact on H2 release, reversibility, and thermodynamics. J. Phys. Chem. C 114, 4675 (2010).
P. Adelhelm and P.E. de Jongh: The impact of carbon materials on the hydrogen storage properties of light metal hydrides. J. Mater. Chem. 21, 2417 (2011).
C.P. Baldé, B.P.C. Hereijgers, J.H. Bitter, and K.P. de Jong: Facilitated hydrogen storage in NaAlH4 supported on carbon nanofibers. Angew. Chem. Int. Ed. 45, 3501 (2006).
A. Züttel, P. Wenger, P. Sudan, P. Mauron, and S. Orimo: Hydrogen density in nanostructured carbon, metals and complex materials. Mater. Sci. Eng., B 108, 9 (2004).
R.K. Bhakta, J.L. Herberg, B. Jacobs, A. Highley, R. Behrens, N.W. Ockwig, J.A. Greathouse, and M.D. Allendorf: Metal-organic frameworks as templates for nanoscale NaAlH4. J. Am. Chem. Soc. 131, 13198 (2009).
V. Stavila, R.K. Bhakta, T.M. Alam, E.H. Majzoub, and M.D. Allendorf: Reversible hydrogen storage by NaAlH4 confined within a titanium-functionalized MOF-74(Mg) nanoreactor. ACS Nano 74, 9807 (2012).
S-I. Orimo, Y. Nakamori, J.R. Eliseo, A. Züttel, and C.M. Jensen: Complex hydrides for hydrogen storage. Chem. Rev. 107, 4111 (2007).
W. Lohstroh, A. Roth, H. Hahn, and M. Fichtner: Thermodynamic effects in nanoscale NaAlH4. Chem. Phys. Chem. 11, 789 (2010).
C.P. Baldé, B.P.C. Hereijgers, J.H. Bitter, and K.P. de Jong: Sodium alanate nanoparticles-linking size to hydrogen storage properties. J. Am. Chem. Soc. 130, 6761 (2008).
Y. Li, G. Zhou, F. Fang, X. Yu, Q. Zhang, L. Ouyang, M. Zhu, and D. Sun: De-/re-hydrogenation features of NaAlH4 confined exclusively in nanopores. Acta Mater. 59, 1829 (2011).
P.E. de Jongh and P. Adelhelm: Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 3, 1332 (2010).
J. Gao, P. Ngene, I. Lindemann, O. Gutfleisch, K.P. de Jong, and P.E. de Jongh: Enhanced reversibility of H2 sorption in nanoconfined complex metal hydrides by alkali metal addition. J. Mater. Chem. 22, 13209 (2012).
P. Adelhelm, J. Gao, M.H.W. Verkuijlen, C. Rongeat, M. Herrich, P.J.M. van Bentum, O. Gutfleisch, A.P.M. Kentgens, K.P. de Jong, and P.E. de Jongh: Comprehensive study of melt infiltration for the synthesis of NaAlH4/C nanocomposites. Chem. Mater. 22, 2233 (2010).
M. Felderhoff, C. Weidenthaler, R. von Helmolt, and U. Eberle: Hydrogen storage: The remaining scientific and technological challenges. Phys. Chem. Chem. Phys. 9, 2643 (2007).
X-F. Lei and J-X. Ma: Synthesis and electrochemical performance of aluminum based composites. J. Braz. Chem. Soc. 21, 209 (2010).
C-M. Park and H-J. Sohn: Novel antimony/aluminum/carbon nanocomposite for high-performance rechargeable lithium batteries. Chem. Mater. 20, 3169 (2008).
A. Chandrasoma, R. Grant, A.E. Bruce, and M.R.M. Bruce: Electrochemical polymerization of aniline on carbon–aluminum electrodes for energy storage. J. Power Sources 219, 285 (2012).
E.C. Ashby, G.J. Brendel, and H.E. Redman: Direct synthesis of complex metal hydrides. Inorg. Chem. 2, 499 (1963).
E.C. Ashby, F.R. Dobbs, and H.P. Hopkins: Composition of complex aluminum hydrides and borohydrides, as inferred from conductance, molecular association, and spectroscopic studies. J. Am. Chem. Soc. 95, 2823 (1972).
J. Wang, A.D. Ebner, and J.A. Ritter: Physiochemical pathway for cyclic dehydrogenation and rehydrogenation of LiAlH4. J. Am. Chem. Soc. 128, 5949 (2006).
S. Jun, S.H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna, and O. Terasaki: Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J. Am. Chem. Soc. 122, 10712 (2000).
K. Pinkert, L. Giebeler, M. Herklotz, S. Oswald, J. Thomas, A. Meier, L. Borchardt, S. Kaskel, H. Ehrenberg, and J. Eckert: Functionalised porous nanocomposites: A multidisciplinary approach to investigate designed structures for supercapacitor applications. J. Mater. Chem. A 1, 4904 (2013).
S. Oswald, K. Nikolowski, and H. Ehrenberg: Quasi in situ XPS investigations on intercalation mechanisms in Li-ion battery materials. Anal. Bioanal. Chem. 393, 1871 (2009).
S. Oswald, K. Nikolowski, and H. Ehrenberg: XPS investigations of valence changes during cycling of LiCrMnO4-based cathodes in Li-ion batteries. Surf. Interface Anal. 42, 916 (2010).
L. Himakumar, B. Viswanathan, and S. Srinivasamurthy: Dehydriding behaviour of LiAlH4—the catalytic role of carbon nanofibres. Int. J. Hydrogen Energy 33, 366 (2008).
J. Graetz, J. Wegrzyn, and J.J. Reilly: Regeneration of lithium aluminum hydride. J. Am. Chem. Soc. 130, 17790 (2008).
M. Dampc, E. Szymańska, B. Mielewska, and M. Zubek: Ionization and ionic fragmentation of tetrahydrofuran molecules by electron collisions. J. Phys. B: At. Mol. Opt. Phys. 44, 055206 (2011).
P.M. Mayer, M.F. Guest, L. Cooper, L.G. Shpinkova, E.E. Rennie, D.M.P. Holland, and D.A. Shaw: Does tetrahydrofuran ring open upon ionization and dissociation? A TPES and TPEPICO investigation. J. Phys. Chem. A 113, 10923 (2009).
Y.J. Choi, J. Lu, Y. Sohn, Z.Z. Fang, C. Kim, R.C. Bowman, and S. Hwang: Reaction mechanisms in the Li3AlH6/LiBH4 and Al/LiBH4 systems for reversible hydrogen storage. Part 2: Solid-state NMR studies. J. Phys.Chem. C 115, 6048 (2011).
M.H.W. Verkuijlen, D. Gelder, P.J.M. Van Bentum, and A.P.M. Kentgens: Oxidation products of NaAlH4 studied by solid-state NMR and X-ray diffraction. J. Phys.Chem. C 115, 7002 (2011).
J.L. Herberg, R.S. Maxwell, and E.H. Majzoub: 27Al and 1H MAS NMR and 27Al multiple quantum studies of Ti-doped NaAlH4. J. Alloys Compd. 417, 39 (2006).
E.H. Majzoub, J.L. Herberg, R. Stumpf, S. Spangler, and R.S. Maxwell: XRD and NMR investigation of Ti-compound formation in solution-doping of sodium aluminum hydrides: Solubility of Ti in NaAlH4 crystals grown in THF. J. Alloys Compd. 394, 265 (2005).
K.D. Moulder, J.F. Stickle, W.F. Sobol, and P.E. Bomben: Handbook of X-ray Photoelectron Spectroscopy (Perkin-Elmer Corp., Eden Prairie, MN, 1992).
P.B. Amama, J.T. Grant, P.J. Shamberger, A.A. Voevodin, and T.S. Fisher: Improved dehydrogenation properties of Ti-doped LiAlH4: Role of Ti precursors. J. Phys. Chem. C 116, 21886 (2012).
G.P. Lopez, D.G. Castner, and B.D. Ratner: XPS O 1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf. Interface Anal. 17, 267 (1991).
K. Kanamura, H. Tamura, and Z. Takehara: XPS analysis of a lithium surface immersed in propylene carbonate solution containing various salts. J. Electroanal. Chem. 333, 127 (1992).
A. Andersson, A. Henningson, H. Siegbahn, U. Jansson, and K. Edström: Electrochemically lithiated graphite characterised by photoelectron spectroscopy. J. Power Sources 119–121, 522 (2003).
J. Clayden and S.A. Yasin: Pathways for decomposition of THF by organolithiums: The role of HMPA. New J. Chem. 26, 191 (2002).
K. Wang and P.N. Ross: XPS and UPS characterization of the reactions of Al(111) with tetrahydrofuran and propylene carbonate. Surf. Sci. 365, 753 (1996).
D. Lacina, L. Yang, I. Chopra, J. Muckerman, Y. Chabal, and J. Graetz: Investigation of LiAlH4-THF formation by direct hydrogenation of catalyzed Al and LiH. Phys. Chem. Chem. Phys. 14, 6569 (2012).
D.E. Bikiel, F. Di Salvo, M.C. González Lebrero, F. Doctorovich, and D.A. Estrin: Solvation and structure of LiAlH(4) in ethereal solvents. Inorg. Chem. 44, 5286 (2005).
S.K. Maity, L. Flores, J. Ancheyta, and H. Fukuyama: Carbon-modified alumina and alumina-carbon-supported hydrotreating catalysts. Ind. Eng. Chem. Res. 48, 1190 (2009).
J. Khom-in, P. Praserthdam, J. Panpranot, and O. Mekasuwandumrong: Dehydration of methanol to dimethyl ether over nanocrystalline Al2O3 with mixed γ- and χ-crystalline phases. Catal. Commun. 9, 1955 (2008).
Q. Hao, Y. Zhao, H. Yang, Z. Liu, and Z. Liu: Alumina grafted to SBA-15 in supercritical CO2 as a support of cobalt for Fischer − Tropsch synthesis. Energy Fuels 26, 6567 (2012).
Acknowledgments
The authors are indebted to the EU (ERSF) and the Free State of Saxony (SAB Grant No. 14227/2337) within the ADDE—Functional structure design of new high performance materials via atomic design and defect engineering (Grant No. 14227/2337) and the European Center for Emerging Materials and Processes Dresden (ECEMP) excellence clusters B1 and D2 (SAB Grant Nos. 100112628 and 100111670) for financial support. The German Federal Ministry of Education and Research (BMBF) is acknowledged for financial support (Grant No. 03KP801) for the JEOL JAMP-9500F auger electron spectrometer. The authors furthermore thank U. Georgi and R.R. Rottenkügler for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Supplementary Material
Supplementary Material
Supplementary materials can be viewed in this issue of the Journal of Materials Research by visiting u]http://journals.cambridge.org/jmr.
Rights and permissions
About this article
Cite this article
Klose, M., Lindemann, I., Minella, C.B. et al. Unusual oxidation behavior of light metal hydride by tetrahydrofuran solvent molecules confined in ordered mesoporous carbon. Journal of Materials Research 29, 55–63 (2014). https://doi.org/10.1557/jmr.2013.199
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2013.199