Abstract
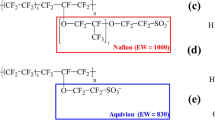
We have used molecular dynamics simulations to examine membrane morphology and the transport of water, methanol, and hydronium in phenylated sulfonated poly(ether ether ketone ketone) (Ph-SPEEKK) and Nafion membranes at 360 K for a range of hydration levels. In Ph-SPEEKK, the average pore diameter is smaller, the sulfonate groups are more closely packed, the hydronium ions are more strongly bound to sulfonate groups, and the diffusion of water and hydronium is slower relative to the corresponding properties in Nafion at comparable hydration levels. The aromatic carbon backbone of Ph-SPEEKK is more rigid and less hydrophobic than the fluorocarbon backbone of Nafion. Water network percolation in Ph-SPEEKK occurs at a hydration level (λ) of ∼8 H2O/SO3−. At λ = 20, water, methanol, and hydronium diffusion coefficients were 1.4 × 10−5, 0.6 × 10−5, and 0.2 × 10−5 cm2/s, respectively. For λ > 20, wide pores develop leading to an increase in methanol crossover and ion transport.















Similar content being viewed by others
References
X. Ren, M.S. Wilson, and S. Gottesfeld: High performance direct methanol polymer electrolyte fuel cells. J. Electrochem. Soc. 143, L12 (1996).
S.K. Kamrudin, F. Achmad, and W.R.W. Daud: Overview of the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 34, 6902 (2009).
A. Heinzel and V.M. Barragan: A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J. Power Sources 84, 70 (1999).
K.A. Mauritz and R.B. Moore: State of understanding of Nafion. Chem. Rev. 104, 4535 (2004).
V. Neburchilov, J. Martin, H. Wang, and J. Zhang: A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 169, 221 (2007).
N.W. Deluca and Y.A. Elabd: Polymer electrolyte membranes for the direct methanol fuel cell: A review. J. Polym. Sci., Part B: Polym. Phys. 44, 2201 (2006).
K.D. Kreuer: On the development of proton conducting polymer membranes for technological applications. Solid State Ionics 97, 1 (1997).
K.D. Kreuer: On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 185, 29 (2001).
R. Devanathan: Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 1, 101 (2008).
B. Smitha, S. Sridhar, and A.A. Khan: Solid polymer electrolyte membranes for fuel cell applications—A review. J. Membr. Sci. 259, 10 (2005).
Q. Li, R. He, J.O. Jensen, and N.J. Bjerrum: Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem. Mater. 15, 4896 (2003).
G. Alberti, M. Casciola, L. Massinelli, and B. Bauer: Polymeric proton conducting membranes for medium temperature fuel cells (110–160 °C). J. Membr. Sci. 185, 73 (2001).
W.H.J. Hogarth, J.C. Diniz da Costa, and G.Q. Lu: Solid acid membranes for high temperature (>140 °C) proton exchange membrane fuel cells. J. Power Sources 142, 223 (2005).
M.A. Hickner, H. Ghassemi, Y.S. Kim, B.R. Einsla, and J.E. McGrath: Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 104, 4587 (2004).
R.W. Kopitzke, C.A. Linkous, H.R. Anderson, and G.L. Nelson: Conductivity and water uptake of aromatic-based proton exchange membrane electrolytes. J. Electrochem. Soc. 147, 1677 (2000).
B. Yang and A. Manthiram: Comparison of the small angle x-ray scattering study of sulfonated poly(etheretherketone) and Nafion membranes for direct methanol fuel cells. J. Power Sources 153, 29 (2006).
B. Yang and A. Manthiram: Sulfonated poly(ether ether ketone) membranes for direct methanol fuel cells. Electrochem. Solid-State Lett. 6, A229 (2003).
R.T.S. Muthulakshmi, V. Choudhary, and I.K. Varma: Sulphonated poly(ether ether ketone): Synthesis and characterisation. J. Mater. Sci. 40, 629 (2005).
Y. Fu, A. Manthiram, and M.D. Guiver: Blend membranes based on sulfonated poly(ether ether ketone) and polysulfone bearing benzimidazole side groups for proton exchange membrane fuel cells. Electrochem. Commun. 8, 1386 (2006).
P. Knauth, E. Sgreccia, A. Donnadio, M. Casciola, and M.L. Di Vona: Water activity coefficient and proton mobility in hydrated acidic polymers. J. Electrochem. Soc. 158, B159 (2011).
B. Liu, G.P. Robertson, D-S. Kim, M.D. Guiver, W. Hu, and Z. Jiang: Aromatic poly(ether ketone)s with pendant sulfonic acid phenyl groups prepared by a mild sulfonation method for proton exchange membranes. Macromolecules 40, 1934 (2007).
B. Liu, Y.S. Kim, W. Hu, G.P. Robertson, B.S. Pivovar, and M.D. Guiver: Homopolymer-like sulfonated phenyl- and diphenyl-poly(arylene ether ketone)s for fuel cell applications. J. Power Sources 185, 899 (2008).
J.A. Elliott, S. Hanna, A.M.S. Elliott, and G.E. Cooley: Atomistic simulation and molecular dynamics of model systems for perfluorinated ionomer membranes. Phys. Chem. Chem. Phys. 1, 4855 (1999).
A. Vishnyakov and A.V. Neimark: Molecular simulation study of Nafion membrane solvation in water and methanol. J. Phys. Chem. B 104, 4471 (2000).
E. Spohr, P. Commer, and A.A. Kornyshev: Enhancing proton mobility in polymer electrolyte membranes: Lessons from molecular dynamics simulations. J. Phys. Chem. B 106, 10560 (2002).
S.S. Jang, V. Molinero, T. Cagin, and W.A. Goddard III: Nanophase-segregation and transport in Nafion 117 from molecular dynamics simulations: Effect of monomeric sequence. J. Phys. Chem. B 108, 3149 (2004).
S. Urata, J. Irisawa, A. Takada, W. Shinoda, S. Tsuzuki, and M. Mikami: Molecular dynamics simulation of swollen membrane of perfluorinated ionomer. J. Phys. Chem. B 109, 4269 (2005).
A. Venkatnathan, R. Devanathan, and M. Dupuis: Atomistic simulations of hydrated Nafion and temperature effects on hydronium ion mobility. J. Phys. Chem. B 111, 7234 (2007).
R. Devanathan, A. Venkatnathan, and M. Dupuis: Atomistic simulation of Nafion membrane. I Effect of hydration on membrane nanostructure. J. Phys. Chem. B 111, 8069 (2007).
R. Devanathan, A. Venkatnathan, and M. Dupuis: Atomistic simulation of Nafion membrane. 2. Dynamics of water molecules and hydronium ions. J. Phys. Chem. B 111, 8069 (2007).
S. Cui, J. Liu, M.E. Selvan, D.J. Keffer, B.J. Edwards, and W.V. Steele: A molecular dynamics study of a Nafion polyelectrolyte membrane and the aqueous phase structure for proton transport. J. Phys. Chem. B 111, 2208 (2007).
S. Cui, J. Liu, M.E. Selvan, S.J. Paddison, D.J. Keffer, and B.J. Edwards: Comparison of the hydration and diffusion of protons in perfluorosulfonic acid membranes with molecular dynamics simulations. J. Phys. Chem. B 112, 13273 (2008).
J. Liu, N. Suraweera, D.J. Keffer, S. Cui, and S.J. Paddison: On the relationship between polymer electrolyte structure and hydrated morphology of perfluorosulfonic acid membranes. J. Phys. Chem. C 114, 11279 (2010).
I.H. Hristov, S.J. Paddison, and R. Paul: Molecular modeling of proton transport in the short-side-chain perfluorosulfonic acid ionomer. J. Phys. Chem. B 112, 2937 (2008).
J. Karo, A. Aabloo, J.O. Thomas, and D. Brandell: Molecular dynamics modeling of proton transport in Nafion and Hyflon nanostructures. J. Phys. Chem. B 114, 6056 (2010).
C.K. Knox and G.A. Voth: Probing selected morphological models of hydrated Nafion using large-scale molecular dynamics simulations J. Phys. Chem. B 114, 3205 (2010).
R. Devanathan, A. Venkatnathan, R. Rousseau, M. Dupuis, T. Frigato, W. Gu, and V. Helms: Atomistic simulation of water percolation and proton hopping in Nafion fuel cell membrane. J. Phys. Chem. B 114, 13681 (2010).
S. Feng and G.A. Voth: Proton solvation and transport in hydrated Nafion. J. Phys. Chem. B 115, 5903 (2011).
G. Brunello, S.G. Lee, S.S. Jang, and Y. Qi: A molecular dynamics simulation study of hydrated sulfonated poly(ether ether ketone) for application to polymer electrolyte membrane fuel cells: Effect of water content. J. Renewable Sustainable Energy 1, 033101 (2009).
G.F. Brunello, W.R. Mateker, S.G. Lee, J.I. Choi, and S.S. Jang: Effect of temperature on structure and water transport of hydrated sulfonated poly(ether ether ketone): A molecular dynamics approach. J. Renewable Sustainable Energy 3, 043111 (2011).
P.V. Komarov, I.N. Veselov, P.P. Chu, P.G. Khalatur, and A.R. Khokhlov: Atomistic and mesoscale simulation of polymer electrolyte membranes based on sulfonated poly(ether ether ketone). Chem. Phys. Lett. 487, 291 (2010).
C.V. Mahajan and V. Ganesan: Atomistic simulations of structure of solvated sulfonated poly(ether ether ketone) membranes and their comparisons to Nafion: I. Nanophase segregation and hydrophilic domains. J. Phys. Chem. B 114, 8357 (2010).
C.V. Mahajan and V. Ganesan: Atomistic simulations of structure of solvated sulfonated poly(ether ether ketone) membranes and their comparisons to Nafion: II. Structure and transport properties of water, hydronium ions, and methanol. J. Phys. Chem. B 114, 8367 (2010).
R.D. Lins, R. Devanathan, and M. Dupuis: Modeling of nanophase structural dynamics of phenylated sulfonated poly ether ether ketone ketone (Ph-SPEEKK) membranes as a function of hydration. J. Phys. Chem. B 115, 1817 (2011).
T.D. Astill: Factors influencing electrochemical properties and performance of hydrocarbon based ionomer PEMFC catalyst layers. Ph.D. Thesis, Simon Fraser University, Burnaby, Canada, 2008.
S.L. Mayo, B.D. Olafson, and W.A. Goddard: DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 94, 8897 (1990).
S.S. Jang, M. Blanco, W.A. Goddard III, G. Caldwell, and R.B. Ross: The source of helicity in perfluorinated N-alkanes. Macromolecules. 36, 5331 (2003).
M. Levitt, M. Hirshberg, R. Sharon, K.E. Laidig, and V. Daggett: Calibration and testing of a water model for simulation of the molecular dynamics of proteins and nucleic acids in solution. J. Phys. Chem. B 25, 5051 (1997).
W.L. Jorgensen, D.S. Maxwell, and J. Tirado-Rives: Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225 (1996).
I.T. Todorov, W. Smith, K. Trachenko, and M.T. Dove: DL_POLY_3: New dimensions in molecular dynamics simulations via massive parallelism. J. Mater. Chem. 16, 1911 (2006).
U. Essmann, L. Perera, M.L. Berkowitz, T. Darden, H. Lee, and L.G. Pedersen: A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577 (1995).
M.C. Payne, M.P. Teter, D.C. Allan, T.A. Arias, and J.D. Joannopoulos: Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 64, 1045 (1992).
H.J.C. Berendsen, J.P.M. Postma, W.F. van Gunsteren, A. DiNola, and J.R. Haak: Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684 (1984).
W. Humphrey, A. Dalke, and K. Schulten: VMD: Visual molecular dynamics. J. Mol. Graphics 14, 33 (1996).
M.D. Rintoul and S. Torquato: Precise determination of the critical threshold and exponents in a 3D continuum percolation model. J. Phys. A: Math. Gen. 30, L585 (1997).
S. Bhattacharya and K.E. Gubbins: Fast method for computing pore size distributions of model materials. Langmuir 22, 7726 (2006).
F. Kappel and A. Kuntsevich: An implementation of Shor’s r-algorithm. Comput. Optim. Appl. 15, 193 (2000).
R. Devanathan and M. Dupuis: Insight from molecular modelling: Does the polymer side chain length matter for transport properties of perfluorosulfonic acid membranes? Phys. Chem. Chem. Phys. (2012, in press).
B. Mecheri, A. D’Epifanio, E. Traversa, and S. Licoccia: Sulfonated polyether ether ketone and hydrated tin oxide proton conducting composites for direct methanol fuel cell applications. J. Power Sources 178, 554 (2008).
Acknowledgment
This work was supported by the US Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, under Contract DE-AC05-76RL01830. It was performed in part using the Molecular Science Computing Facility in the EMSL, a national scientific user facility sponsored by DOE’s Office of Biological and Environmental Research located at Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for DOE. This work benefited from resources of the National Energy Research Scientific Computing Center, which is supported by the Office of Science of DOE under Contract No. DE-AC02-05CH1123.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devanathan, R., Idupulapati, N. & Dupuis, M. Molecular modeling of the morphology and transport properties of two direct methanol fuel cell membranes: Phenylated sulfonated poly(ether ether ketone ketone) versus Nafion. Journal of Materials Research 27, 1927–1938 (2012). https://doi.org/10.1557/jmr.2012.165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2012.165