Abstract
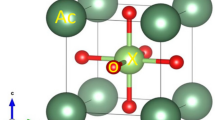
The enthalpies of the monoclinic to orthorhombic transition for a series of A2M3O12 (A = Al, Cr, Fe, In, and Sc; M = Mo or W) compounds were measured by differential scanning calorimetry, and entropies of transition were estimated. The enthalpies of formation from the binary oxides at 25 °C for several A2M3O12 samples were obtained from drop solution calorimetry in molten 3Na2O·4MoO3 at 702 °C. The monoclinic and orthorhombic phases of Sc2Mo3O12 and Sc2W3O12 are the only phases that are enthalpically stable under ambient conditions. The enthalpies of formation from the oxides (ΔHf,ox) for orthorhombic Sc2Mo3O12 and Sc2W3O12 are −47.2 ± 2.1 kJ/mol and −8.5 ± 2.7 kJ/mol, respectively. For Fe2Mo3O12, In2Mo3O12, and In2W3O12, ΔHf,ox values are 51.5 ± 4.5, 7.4 ± 2.9, and 44.5 ± 2.3 kJ/mol, respectively. These phases are entropically stabilized and/or metastable. Enthalpies of formation for phases that could not be measured by calorimetry have been estimated from the enthalpies of transition or trends in the enthalpies of formation. In general, the monoclinic phase is slightly enthalpically stabilized over the orthorhombic phase, while transition to the orthorhombic phase is entropically favored. This confirms that the orthorhombic phase is stable at high temperatures, the monoclinic is stable at low temperatures, and the monoclinic to orthorhombic transition is reversible.









Similar content being viewed by others
References
A.W. Sleight: Negative thermal expansion materials. Curr. Opin. Solid State Mater. Sci. 3, 128 1998
A.W. Sleight: Isotropic negative thermal expansion. Ann. Rev. Mater. Sci. 28, 29 1998
J.S.O. Evans, T.A. Mary A.W. Sleight: Negative thermal expansion materials. Phys. B (Amsterdam) 241–243, 311 1998
J.S.O. Evans: Negative thermal expansion materials. J. Chem. Soc., Dalton Trans. 3317 1999
J.S.O. Evans, T.A. Mary A.W. Sleight: Negative thermal expansion in a large molybdate and tungstate family. J. Solid State Chem. 133, 580 1997
J.S.O. Evans, T.A. Mary A.W. Sleight: Negative thermal expansion in Sc2(WO4)3.J. Solid State Chem. 137, 148 1998
P.M. Forster, A. Yokochi A.W. Sleight: Enhanced negative thermal expansion in Lu2W3O12.J. Solid State Chem. 140, 157 1998
N. Imanaka, M. Hiraiwa, G. Adachi, H. Dabkowska A. Dabkowski: Thermal contraction in Al2(WO4)3 single crystal. J. Cryst. Growth 220, 176 2000
D.A. Woodcock, P. Lightfoot C. Ritter: Negative thermal expansion in Y2(WO4)3.J. Solid State Chem. 149, 92 2000
N. Imanaka G-y. Adachi: Rare earth contribution in solid state electrolytes, especially in the chemical sensor field. J. Alloys Compd. 250, 492 1997
Y. Okazaki, T. Ueda, S. Tamura, N. Imanaka G. Adachi: Trivalent Sc3+ ion conduction in the Sc2(WO4)3-Sc2(MoO4)3 solid solution. Solid State Ionics 136–137, 437 2000
G. Adachi, N. Imanaka S. Tamura: Rare earth ion conduction in solids. J. Alloys Compd. 323–324, 534 2001
N. Imanaka G-Y. Adachi: Rare earth ion conduction in tungstate and phosphate solids. J. Alloys Compd. 344, 137 2002
N. Imanaka, Y. Kobayashi G-y. Adachi: A direct evidence for trivalent ion conduction in solids. Chem. Lett. (Jpn.) 6, 433 1995
E. Gallucci, C. Goutaudier, F. Bourgeois, G. Boulon M.T. Cohen-Adad: Comprehensive study of third-order nonlinear tungstates: Relationship between structural and vibrational properties in Raman shifters. J. Solid State Chem. 163, 506 2002
R.A. Secco, H. Liu, N. Imanaka G. Adachi: Pressure-induced amorphization in negative thermal expansion Sc2(WO4)3. J. Mater. Sci. Lett. 20, 1339 2001
R.A. Secco, H. Liu, N. Imanaka G. Adachi: Anomalous ionic conductivity of Sc2(WO4)3 mediated by structural changes at high pressures and temperatures. J. Phys. Condens. Matter 14, 11285 2002
R.A. Secco, H. Liu, N. Imanaka, G. Adachi M.D. Rutter: Electrical conductivity and amorphization of Sc2(WO4)3 at high pressures and temperatures. J. Phys. Chem. Solids 63, 425 2002
T. Varga, A.P. Wilkinson, C. Lind, W.A. Bassett C-S. Zha: In situ high-pressure synchrotron x-ray diffraction study of Sc2W3O12 at up to 10 GPa. Phys. Rev. B 71, 214106 2005
T. Varga, A.P. Wilkinson, J.D. Jorgensen S. Short: Neutron powder diffraction study of the orthorhombic to monoclinic transition in Sc2W3O12 on compression. Solid State Sci. 8, 289 2006
M.T. Weller, P.F. Henry C.C. Wilson: An analysis of the thermal motion in the negative thermal expansion material Sc2(WO4)3 using isotopes in neutron diffraction. J. Phys. Chem. B 104, 12224 2000
M. Maczka, K. Hermanowicz J. Hanuza: Phase transition and vibrational properties of A2(BO4)3 compounds (A = Sc, In; B = Mo, W). J. Molec. Struct. 744–747, 283 2005
N. Garg, C. Murli, A.K. Tyagi S.M. Sharma: Phase transitions in Sc2(WO4)3 under high pressure. Phys. Rev. B 72, 064106 2005
J.S.O. Evans T.A. Mary: Structural phase transitions and negative thermal expansion in Sc2(MoO4)3. Int. J. Inorg. Mater. 2, 143 2000
W. Paraguassu, M. Maczka, A.G. Souza Filho, P.T.C. Freire, J. Mendes Filho, F.E.A. Melo, L. Macalik, L. Gerward, J. Staun Olsen, A. Waskowska J. Hanuza: Pressure-induced structural transformations in the molybdate Sc2(MoO4)3. Phys. Rev. B 69, 094111(1 2004
A.K. Arora, R. Nithya, T. Yagi, N. Miyajima T.A. Mary: Two-stage amorphization of scandium molybdate at high pressure. Solid State Commun. 129, 9 2004
T. Varga, A.P. Wilkinson, C. Lind, W.A. Bassett C-S. Zha: High pressure synchrotron x-ray powder diffraction study of Sc2Mo3O12 and Al2W3O12. J. Phys. Condens. Matter 17, 4271 2005
A.K. Arora, T. Yagi, N. Miyajima T.A. Mary: Amorphization and decomposition of scandium molybdate at high pressure. J. Appl. Phys. 97, 013508 2005
T.R. Ravindran, V. Sivasubramanian A.K. Arora: Low temperature Raman spectroscopic study of scandium molybdate. J. Phys. Condens. Matter 17, 277 2005
A.K. Tyagi, S.N. Achary M.D. Mathews: Phase transition and negative thermal expansion in A2(MoO4)3 system (A = Fe3+, Cr3+ and Al3+). J. Alloys Compd. 339, 207 2002
S.N. Achary, G.D. Mukherjee, A.K. Tyagi S.N. Vaidya: Preparation, thermal expansion, high pressure and high temperature behavior of Al2(WO4)3. J. Mater. Sci. 37, 2501 2002
G.D. Mukherjee, S.N. Achary, A.K. Tyagi S.N. Vaidya: High pressure AC resistivity and compressibility study on Al2(WO4)3. J. Phys. Chem. Solids 64, 611 2003
M. Maczka, W. Paraguassu, A.G. Souza Filho, P.T.C. Freire, J. Mendes Filho, F.E.A. Melo J. Hanuza: High-pressure Raman study of Al2(WO4)3. J. Solid State Chem. 177, 2002 2004
G.D. Mukherjee, V. Vijaykumar, S.N. Achary, A.K. Tyagi B.K. Godwal: Phase transitions in Al2(WO4)3: High pressure investigations of low frequency dielectric constant and crystal structure. J. Phys. Condens. Matter 16, 7321 2004
N. Garg, V. Panchal, A.K. Tyagi S.K. Sharma: Pressure-induced phase transitions in Al2(WO4)3. J. Solid State Chem. 178, 998 2005
K. Aizu: Possible species of ferromagnetic, ferroelectric, and ferroelastic crystals. Phys. Rev. B 2, 754 1970
K. Nassau, H.J. Levinstein G.M. Loiacono: Trivalent rare-earth tungstates of the type M2(WO4)3. J. Am. Ceram. Soc. 47, 363 1964
K. Nassau, H.J. Levinstein G.M. Loiacono: A comprehensive study of trivalent tungstates and molybdates of the type L2(MO4)3. J. Phys. Chem. Solids 26, 1805 1965
A.W. Sleight L.H. Brixner: A new ferroelastic transition in some A2(MO4)3 molybdates and tungstates. J. Solid State Chem. 7, 172 1973
D.A. Fleming, D.W. Johnson P.J. Lemaire: Article comprising a temperature compensated optical fiber refractive index grating, U.S. Patent No. 5 694 503 (1997)
C. Verdon D.C. Dunand: High-temperature reactivity in the ZrW2O8–Cu system. Scripta Mater. 36, 1075 1997
D.A. Fleming, P.J. Lemaire D.W. Johnson: Temperature compensated optical fiber refractive index grating. European Patent 97-306798, 19970902 (1998)
H. Holzer D.C. Dunand: Phase transformation and thermal expansion of Cu/ZrW2O8 metal matrix composites. J. Mater. Res. 14, 780 1999
D.K. Balch D.C. Dunand: Copper-zirconium tungstate composites exhibiting low and negative thermal expansion influenced by reinforcement phase transformations. Metall. Mater. Trans. A 35A, 1159 2004
V.M. Amosov V.E. Plyushchev: Thermochemistry of tungstates of scandium subgroup elements. Neorg. Mater. 4, 1309 1968
L.A. Reznitskii: Enthalpy factor of stabilization and a high cationic conductivity of molybdates M2(MoO4)3 with Sc2(WO4)3-type structure. Zh. Fizicheskoi Khimii 76, 1528 2002
V. Sivasubramanian, T.R. Ravindran A.K. Arora: Structural phase transition in indium tungstate. J. Appl. Phys. 96, 387 2004
A. Navrotsky: Progress and new directions in high temperature calorimetry. Phys. Chem. Miner. 2, 89 1977
A. Navrotsky: Progress and new directions in high temperature calorimetry revisited. Phys. Chem. Miner. 24, 222 1997
A. Navrotsky O.J. Kleppa: A calorimetric study of molten Na2MoO4–MoO3 mixtures at 970 °K. Inorg. Chem. 6, 2119 1967
J.H. Cheng A. Navrotsky: Enthalpies of formation of LaBO3 perovskites (B = Al, Ga, Sc, and In). J. Mater. Res. 18, 2501 2003
R.D. Shannon: Revised effective ionic radii and systematic studies of interatomic distancesin halides and chalcogenides. Acta Crystallogr., Sect. A 32, 751 1976
WebElements, http://www.webelements.com, (University of Sheffield and WebElements Ltd., Sheffield, UK, 1993–2007): Accessed April 2, 2007.
A. Navrotsky: Repeating patterns in mineral energetics. Am. Mineral. 79, 589 1994
T. Varga, C. Lind, A.P. Wilkinson, H. Xu, C.E. Lesher A. Navrotsky: Heats of formation for several crsytalline polymorphs and pressure-induced amorphous forms of AM2O8 (A = Zr, Hf) and ZrW2O8. Chem. Mater. 19, 468 2007
JADE: (Materials Data, Inc., Livermore, CA, 2002)
A.L. Allred E.G. Rochow: A scale of electronegativity based on electrostatic force. J. Inorg. Nucl. Chem. 5, 264 1958
W.T.A. Harrison: Crystal structures of paraelastic aluminum molybdate and ferric molybdate, β–Al2(MoO4)3 and β–Fe2(MoO4)3. Mater. Res. Bull. 30, 1325 1995
J. Majzlan, A. Navrotsky B.J. Evans: Thermodynamics and crystal chemistry of the hematite-corundum solid solution and the FeAlO3 phase. Phys. Chem. Miner. 29, 515 2002
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melançon, A.D. Pelton S. Petersen: FactSage thermochemical software and databases. CALPHAD 26, 189 2002
M.R. Ranade, F. Tessier, A. Navrotsky R. Marchand: Calorimetric determination of the enthalpy of formation of InN and comparison with AlN and GaN. J. Mater. Res. 16, 2824 2001
Acknowledgment
This work was partly supported by the National Science Foundation under Grant No. DMR 06-01892.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varga, T., Moats, J.L., Ushakov, S.V. et al. Thermochemistry of A2M3O12 negative thermal expansion materials. Journal of Materials Research 22, 2512–2521 (2007). https://doi.org/10.1557/jmr.2007.0311
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2007.0311