Abstract
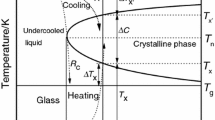
If a metal contracts upon solidification, the specific volume of a metallic liquid phase must not be smaller than that of the corresponding crystal. As molten metals have higher thermal expansion coefficients compared with those of the corresponding crystals, the intersection point of two specific-volume–temperature plots of the liquid and the corresponding solid crystalline phase by analogy with Kauzmann’s paradox for entropy could be treated as an ideal glass-transition temperature. This paper describes this phenomenon observed for a number of pure metals and gives a semiempirical criterion for the achievement of a good glass-forming ability.
Similar content being viewed by others
Change history
01 August 2007
An Erratum to this paper has been published: https://doi.org/10.1557/JMR.2007.0167e
References
P.G. Debenedetti and F.H. Stillinger: Supercooled liquids and the glass transition. Nature 410, 259 (2001).
D. Turnbull: Under what conditions can a glass be formed? Contemp. Phys. 10, 473 (1969).
A. Van den Beukel and J. Sietsma: The glass transition as a free volume related kinetic phenomenon. Acta Metall. Mater. 38, 383 (1990).
D. Turnbull and M.H. Cohen: On the free-volume model of the liquid–glass transition. J. Chem. Phys. 52, 3038 (1970).
T.G. Fox and P.J. Flory: Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 21, 581 (1950).
M.H. Cohen and D. Turnbull: Molecular transport in liquids and glasses. J. Chem. Phys. 31, 1164 (1959).
M.H. Cohen and G.S. Grest: Liquid-glass transition, a free-volume approach. Phys. Rev. 20, 1077 (1979).
R. Brüning and K. Samwer: Glass transition on long time scales. Phys. Rev. B 46, 11318 (1992).
W. Kauzmann: The nature of the glassy state and the behavior of liquids at low temperatures. Chem. Rev. 43, 219 (1948).
A.R. Yavari: Small volume change on melting as a new criterion for easy formation of metallic glasses. Phys. Lett. A. 95, 165 (1983).
W.F. Gale and T.C. Totemeier: Smithells Metals Reference Book, 8th edition, (Elsevier Butterworth-Heinemann Ltd., Oxford UK, 2004), p. 14–1.
Y.S. Touloukian, R.K. Kirby, R.E. Taylor, and P.D. Desai: Thermophysical properties of matter, in Thermal Expansion, Metallic Elements and Alloys, Vol. 12, (IFI/Plenum, New York, NY and Washington, DC, 1975), pp. 1–100.
V.G. Bar’yakhtar, L.E. Mikhalova, A.G. Il’inski, A.V. Romanova, and T.M. Khristenko: Thermal expansion mechanism of liquid metals. Sov. Phys. JETP 68, 811 (1989).
W.F. Gale and T.C. Totemeier: Smithells Metals Reference Book, 8th edition, (Elsevier Butterworth-Heinemann Ltd., Oxford UK, 2004), pp. 14–10.
W. Coy and R. Mateer: Density of molten aluminum by maximum bubble pressure method. Trans. ASM 58, 99 (1965).
S. Saito, Y. Shiraishi, and Y. Sakuma: Density measurement of molten metals by levitation technique at temperatures between 1800° and 2200 °C. Trans. Iron Steel Inst. Jpn. 9, 118 (1969).
S. Watanabe: Densities and viscosities of iron, cobalt and Fe–Co alloy in liquid state. Trans. Jpn. Inst. Metals 12, 17 (1971).
J. Brillo and I. Egry: Density determination of liquid copper, nickel, and their alloys. Int. J. Thermophys. 24, 1155 (2003).
D.J. Steinberg: A simple relationship between the temperature dependence of the density of liquid metals and their boiling temperatures. Metall. Trans. 5, 1341 (1974).
D.V. Louzguine, A.R. Yavari, K. Ota, G. Vaughan, and A. Inoue: Synchrotron x-ray radiation diffraction studies of thermal expansion, free volume change and glass transition phenomenon in Cu-based glassy and nanocomposite alloys on heating. J. Non-Cryst. Solids 351, 1639 (2005).
A.L. Greer: Thermodynamics of undercooled liquids. J. Less Common Met. 145, 131 (1988).
A. Inoue, T. Negishi, H.M. Kimura, T. Zhang, and A.R. Yavari: High packing density of Zr- and Pd-based bulk amorphous. Alloys Mater. Trans., JIM 39, 318 (1998).
L. Battezzati and M. Baricco: Analysis of volume effects in metallic glass formation. J. Less Common Met. 145, 31 (1988).
H.S. Chen: Thermodynamic considerations on the formation and stability of metallic glasses. Acta Metall. 22, 1505 (1974).
A. Inoue: Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 48, 279 (2000).
D.V. Louzguine-Luzgin, A.D. Setyawan, H. Kato, and A. Inoue: Influence of thermal conductivity on the glass-forming ability of Ni-based and Cu-based alloys. Appl. Phys. Lett. 88, 251902 (2006).
J.H. Perepezko and R.J. Hebert: Amorphous aluminum alloys—Synthesis and stability. J. Metall. 54, 34 (2002).
A. Inoue: High-strength bulk amorphous-alloys with low critical cooling rates. Mater. Trans., JIM 36, 866 (1995).
V.A. Shneidman and D.R. Uhlmann: The fast cooling/heating rate effects in devitrification of glasses. II. Crystallization kinetics. J. Chem. Phys. 109, 186 (1998).
W. Klement, R.H. Willens, and P. Duwez: Non-crystalline structure in solidified gold–silicon alloys. Nature 187, 869 (1960).
D. Turnbull and M.H. Cohen: Free-volume model of the amorphous phase: Glass transition. J. Chem. Phys. 34, 120 (1961).
D.V. Louzguine-Luzgin and A. Inoue: Nano-devitrification of glassy alloys. J. Nanosci. Nanotechnol. 5, 999 (2005).
Z.P. Lu and C.T. Liu: A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 50, 3501 (2002).
A.L. Greer: Metallic glasses. Science 267, 1947 (1995).
W.L. Johnson: Bulk glass-forming metallic alloys: Science and technology. MRS Bull. 24, 42 (1999).
T. Ichitsubo, E. Matsubara, H. Numakura, K. Tanaka, N. Nishiyama, and R. Tarumi: Glass-liquid transition in a less-stable metallic glass. Phys. Rev. B 72, 052201 (2005).
C.A. Angell: Formation of glasses from liquids and biopolymers. Science 267, 1924 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Louzguine-Luzgin, D.V., Inoue, A. An extended criterion for estimation of glass-forming ability of metals. Journal of Materials Research 22, 1378–1383 (2007). https://doi.org/10.1557/jmr.2007.0167
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2007.0167