Conclusions
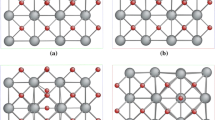
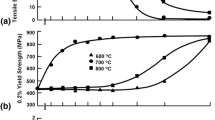
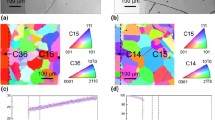
The calculated lattice energies apparently reflect the hydration susceptibility as a function of dopant species. While the experimental work done in this area is limited, what is reported in the literature supports the present work. The calculated lattice energies are also a function of dopant concentration. Experimental results would be useful in determining the accuracy of this observation. A degree of ordering has been imposed due to the relatively small supercell used in this study. The 2×2×2 models used in these studies imposed a high degree of order on the defects in the system. We are in the process of investigating the dependence of doped lattice energy estimates on the size of the supercell. We plan to use a supercell which contains 64 unit cells, which will open up the number of possible defect configurations. We also plan to correlate future results to systematic measurements of hydration susceptibility, sintering behavior, surface microhardness, as well as elasticity and strength properties. A more accurate portrayal of these structures will require a significant amount of computing time. Indications are that these types of studies can be profitable.
Similar content being viewed by others
References
M.J. Gildersleeve and R.J. Brook, “Fast Firing of Sea-Water Magnesia Materials,” Trans J. Brit. Ceram. Soc. 83 (1984) p. 154–157.
B.L. Fletched, J.R. Stevenson, and A. Whitaker, “Phase Equilibria in the System CaO-MgO-B2O3 at 900°C,” Trans J. Brit. Ceram. Soc. 69 (1970) p. 95–97.
H.J.S. Kriek, W.F. Ford, and J. White, “The Effect of Additions on the Sintering and Dead-Burning of Magnesia,” Trans. Brit. Ceram. Soc. 58 (1959) p. 1–34.
E.A. Hyleraas, “Gleichgewichtslage der Atome, Doppelbrechung und Optisches Drehungsvermogen von β-Quartz,” Z. Physik 44 (1927) p. 871–876.
Q.C. Johnson and D.H. Templeton, “Madelung Constants for Several Structures,” J. Chem. Phys. 34 (1961) p. 2004–2007.
R.F. Giese, Jr., “Electrostatic Energy of Columbite and Ixiolite,” Nature 256 p. 31–32.
G.E. Brown and P.M. Fenn, “Structure Energies of the Alkali Feldspars,” Physics and Chemistry of Minerals 4 (1979) p. 83–100.
J.E. Post and C.W. Burnham, “Disordering on High Albite-Insights from Electrostatic Energy Minimizations,” Geological Soc. of America Absts. with Programs 16 (1984) p. 625.
J.E. Post and C.W. Burnham, “Ionic Modeling of Mineral Structures and Energies in the Electron Gas Approximarion-TiO2 Polymorphs, Quartz, Forsterite, Diopside,” American Mineralogist 71 (1986) p. 142–150.
Chamberlain, “Scapolite-Alkali Atom Configurations, Antiphase Domains, and Compositional Variations,” American Mineralogist 70 (1985) p. 134–140.
R.E. Cohen and C.W. Burnham, “Energetics of Ordering in Aluminous Pyroxines,” American Mineralogist 70 (1985) p. 559–567.
J.E. Post and C.W. Burnham, “Modeling Tunnel-Cation Displacements in Hollandites Using Structure-Energy Calculations,” American Mineralogist 71 (1986) p. 1178–1185.
W.R. Busing, “WMIN, A Computer Program to Model Molecules and Crystals in Terms of Potential Energy Functions,” U.S. National Technical Information Service, ORNL-5747.
F. Bertaut, “L’Energie Electrostatique de Reseaux Ioniques,” J. Phys. Radium 13 (1952) p. 499–505.
C. Kittel, Introduction to Solid State Physics, 5th ed. (1976) p. 88.
T.L. Gilbert, “Soft-Sphere Model for Closed Shell Atoms and Ions,” J. Chem. Phys. 49 (1968) p. 2640.
P. Kofstad, Nonstoichiometry, Diffusion, and Electrical Conductivity in Binary Metal Oxides (Wiley Interscience, John Wiley & Sons, NY, 1972).
C. Butterfield and E.H. Carlson, “Ionic Soft Sphere Parameters from Hartree-Fock-Slater Calculations,” J. Chem. Phys. 56 (1972) p. 4907–4911.
G.K. Layden and M.C. McQurrie, “Effect of Minor Additions on Sintering of MgO,” J. Amer. Ceram. Soc. 42(2) (1959) p. 89–92.
J.W. Nelson and I.B. Cutler, “Effect of Oxide Additions on Sintering of MgO,” J. Amer. Ceram. Soc. 41(10) (1958) p. 406–409.
Rights and permissions
About this article
Cite this article
Howard, S.A., Lee, SH. & Moore, R.E. Using Lattice Energies to Model the Physical/Chemical Behavior of a Doped Refractory Oxide. MRS Bulletin 14, 60–64 (1989). https://doi.org/10.1557/S0883769400061224
Published:
Issue Date:
DOI: https://doi.org/10.1557/S0883769400061224