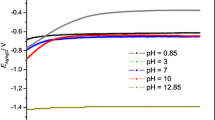
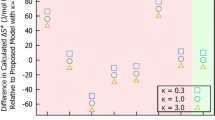
Conclusions
The interactions of solutes with mineral surfaces are central to models of natural mineral corrosion rates and for predictions about the migration rates of contaminants in groundwater. Because of the scale of geologic processes, these predictions must span many decades and potentially large variations in natural chemistry. If these predictions are to gain credibility, details of the reactions must be confirmed at a fine scale. With new methods of surface analysis, important assumptions in the models can be independently verified. The results so far have often confirmed longheld opinions about the control of mineral dissolution and growth, such as the importance of surface microtopography, and have uncovered wholly new phenomena, as well.
Similar content being viewed by others
References
J.I. Drever, The Geochemistry of Natural Waters, 2nd ed. (Prentice-Hall, 1988) p. 269.
V. Pye, and J. Kelley, Chapter 1 in Groundwater Contamination (National Academy Press, Washington, DC, 1984).
L.G.J. Fokkink, PhD thesis, Wageningen University, 1987.
G.A. Parks, in Reviews in Mineralogy #23: Mineral-Water Interface Geochemistry; edited by M.F. Hochella and A.F. White (Mineralogical Society of America, Washington, DC, 1991), p. 133–175; J.A. Davis, and J.A. and D.B. Kent, ibid., p. 177–260.
V.S. Tripathi, PhD thesis, Stanford University, 1983.
R.G. Wilkens, The Study of Kinetics and Mechanism of Reactions of Transition Metal Complexes (Allyn and Bacon, Boston, 1974) p. 81.
A. Blum and A.C. Lasaga, Nature331 (1988) p. 431.
W.H. Casey, J. Colloid Interf. Sci.146 (1991) p. 586.
G.E. Brown, in Reviews in Mineralogy #5: Orthosilicates, edited by P. Ribbe (Mineralogical Society of America, Washington, DC, 1982), p. 275.
J. Burgess, Ions in Solution (John Wiley, New York, 1988) p. 40.
W.H. Casey and H.R. Westrich, Nature355 (1992) p. 157.
W.H. Casey, H.R. Westrich, G.W. Arnold, and J.E. Banfield, Geochim. Cosmochim. Acta53 (1989) p. 821.
J.-C. Petit, J.-C. Dran, and G. Delia Mea, Nature344 (1990) p. 621.
R. Hellmann, C.M. Eggleston, M.F. Hochella, and D.A. Crerar, Geochim. Cosmochim. Acta54 (1990) p. 1267.
J.F. Banfield, D.R. Veblen, and B.F. Jones, Contrib. Mineral. Petrol.106 (1990) p. 110.
I.J. Muir, G.M. Bancroft, and H.W. Nesbitt, Geochim. Cosmochim. Acta53 (1988) p. 1235.
M.F. Hochella Jr., in Reviews in Mineralogy #23: Mineral-Water Interface Geochemistry, edited by M.F. Hochella and A.F. White (Mineralogical Society of America, Washington, DC, 1990) p. 87.
G.E. Brown, in Reviews in Mineralogy #23: Mineral-Water Interface Geochemistry, edited by M.F. Hochella and A.F. White (Mineralogical Society of America, Washington, DC, 1990) p. 309.
K.F. Hayes, A.L. Roe, G.E. Brown, K.O. Hodgson, J.O. Leckie, and G.A. Parks, Science238 (1987) p. 783.
C.M. Eggleston, M.F. Hochella Jr., and G.A. Parks (1990) Geological Society of America Abst. with Programs, A292.
P.A. Johnsson, C.M. Eggleston, and M.E. Hochella Jr., American Mineral.76 (1991) p. 1443.
A.J. Gratz, S. Manne, and P.K. Hansma, Science251 (1991) p. 1343.
P.E. Hillner, A.J. Gratz, S. Manne, and P.K. Hansma, Geology (1992) (submitted).
M.F. Hochella Jr., C.M. Eggleston, V.B. Elings, and M. Thompson, American Mineral.75 (1990) p. 723.
H. Hartman, G. Sposito, A. Yang, S. Manne, S.A.C. Gould, and P.K. Hansma, Clays Clay Minerals38 (1990) p. 337.
H. Lindgreen, J. Garnaes, P.L. Hansen, F. Besenbacher, E. Laegsgaard, I. Stensgaard, S.A. Gould, and P.K. Hansma, American Mineral.76 (1991) p. 1218.
A.L. Weisenhorn, J.E. MacDougall, S.A. Gould, S.D. Cox, W.S. Wise, J. Massie, P. Maivald, V.B. Elings, G.D. Stucky, and P.K. Hansma, Science247 (1990) p. 1330.
C.M. Eggleston, and M.F. Hochella Jr., Geochim. Cosmochim. Acta54 (1990) p. 1511.
C.M. Eggleston, and M.F. Hochella Jr., Science254 (1991) p. 983.
Rights and permissions
About this article
Cite this article
Casey, W.H., Eggleston, C., Johnsson, P.A. et al. Aqueous Surface Chemistry and Corrosion of Minerals. MRS Bulletin 17, 23–29 (1992). https://doi.org/10.1557/S0883769400041245
Published:
Issue Date:
DOI: https://doi.org/10.1557/S0883769400041245