Abstract
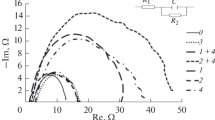
Alloy 22 may be susceptible to crevice corrosion in chloride-containing environments, especially at temperatures above ambient. The presence of oxyanions, especially nitrate, minimizes or eliminates the susceptibility of Alloy 22 to crevice corrosion. Other anions such as sulfate, carbonate and fluoride were also reported as inhibitors of crevice corrosion in Alloy 22. It is argued that the occurrence of crevice corrosion is due to the formation of hydrochloric acid solution in the creviced region. Inhibitors act by eliminating the occurrence of hydrochloric acid or by hampering its action.
Similar content being viewed by others
References
ASTM International, Standard B575, Vol. 02.04 (ASTM, 2002: West Conshohocken, PA).
R. B. Rebak and J. H. Payer, “Passive Corrosion Behavior of Alloy 22,” International High-Level Radioactive Waste Management Conference, Las Vegas, NV April 30 to May 04, 2006 (American Nuclear Society 2006: La Grange Park, IL).
B. A. Kehler, G. O. Ilevbare and J. R. Scully, Corrosion, 1042 (2001).
K. J. Evans and R. B. Rebak “Passivity of Alloy 22 in Concentrated Electrolytes. Effect of Temperature and Solution Composition,” in Corrosion Science – A Retrospective and Current Status in Honor of Robert P. Frankenthal, PV 2002–13, p. 344–354 (The Electrochemical Society, 2002: Pennington, NJ).
K. J. Evans, S. D. Day, G. O. Ilevbare, M. T. Whalen, K. J. King, G. A. Hust, L. L. Wong, J. C. Estill and R. B. Rebak, “Anodic Behavior of Alloy 22 in Calcium Chloride and in Calcium Chloride plus Calcium Nitrate Brines,” in PVP-Vol. 467, Transportation, Storage and Disposal of Radioactive Materials – 2003, p. 55 (ASME, 2003: New York, NY).
C. S. Brossia, L. Browning, D. S. Dunn, O. C. Moghissi, O. Pensado and L. Yang, “Effect of Environment on the Corrosion of Waste Package and Drip Shield Materials,” Publication of the Center for Nuclear Waste Regulatory Analyses (CNWRA 2001–03), September 2001.
D. S. Dunn, L. Yang, Y.-M. Pan and G. A. Cragnolino, “Localized Corrosion Susceptibility of Alloy 22,” Paper 03697 (NACE International, 2003: Houston, TX).
K. J. Evans, A. Yilmaz, S. Daniel Day, L. L. Wong, J. C. Estill and R. B. Rebak, “Using Electrochemical Methods to Determine Alloy 22’s Crevice Corrosion Repassivation Potential,” Journal of Metals, Vol 57, pp. 56–61 (2005).
D. S. Dunn, Y.-M. Pan, L. Yang and G. A. Cragnolino, Corrosion, 61, 1078 (2005).
D. S. Dunn, Y.-M. Pan, L. Yang and G. A. Cragnolino, Corrosion, 62, 3 (2006).
G. A. Cragnolino, D. S. Dunn and Y.-M. Pan, “Localized Corrosion Susceptibility of Alloy 22 as a Waste Package Container Material,” in Scientific Basis for Nuclear Waste Management XXV, Vol. 713, p. 53 (Materials Research Society 2002: Warrendale, PA).
D. S. Dunn and C. S. Brossia, “Assessment of Passive and Localized Corrosion Processes for Alloy 22 as a High-Level Nuclear Waste Container Material,” Paper 02548 (NACE International, 2002: Houston, TX).
J. H. Lee, T. Summers and R. B. Rebak, “A Performance Assessment Model for Localized Corrosion Susceptibility of Alloy 22 in Chloride Containing Brines for High Level Nuclear Waste Disposal Container,” Paper 04692 (NACE International, 2004: Houston, TX).
D. S. Dunn, L. Yang, C. Wu and G. A. Cragnolino, “Effect of Inhibiting Oxyanions on the Localized Corrosion Susceptibility of Waste Package Container Materials,” in Scientific Basis for Nuclear Waste Management XXVIII, Vol. 824 p. 33 (MRS, 2004: Warrendale, PA).
D. S. Dunn, Y.-M. Pan, K. T. Chiang, L. Yang and G. A Cragnolino and X. He, “Localized Corrosion Resistance and Mechanical Properties of Alloy 22 Waste Package Outer Containers,” JOM, January 2005, pp 49–55 (2005).
R. B. Rebak, “Factors Affecting the Crevice Corrosion Susceptibility of Alloy 22,” Paper 05610, Corrosion/2005 (NACE International, 2005: Houston, TX).
D. S. Dunn, Y.-M. Pan, L. Yang and G. A Cragnolino, Corrosion, 61, 11, 1076, 2005.
G. O. Ilevbare, K. J. King, S. R. Gordon, H. A. Elayat, G. E. Gdowski and T. S. E. Summers, Journal of The Electrochemical Society, 152, 12, B547–B554, 2005.
G. O. Ilevbare, R. A. Etien, J. C. Estill, G. A. Hust, A. Yilmaz, M. L. Stuart, and R. B. Rebak, “Anodic Behavior of Alloy 22 in High Nitrate Brines at Temperatures Higher than 100°C,” Paper 93423 in the Proceedings of PVP2006-ICPVT-11, 2006 ASME Pressure Vessels and Piping Division Conference, July 23–27, 2006, Vancouver, BC, Canada.
G. O. Ilevbare, Corrosion, 62, 340 (2006).
R. M. Carranza, M. A. Rodriguez and R. B. Rebak, “Inhibition of Chloride Induced Crevice Corrosion in Alloy 22 by Fluoride Ions,” Paper 06622, Corrosion/2006, NACE International, March 12–16, 2006, San Diego, CA (NACE International, Houston, TX).
M. G. Fontana, Corrosion Engineering, p. 53 (McGraw-Hill, 1986: New York, NY).
A. J. Sedriks, Corrosion of Stainless Steels, p. 177 (John Wiley & Sons, 1996: New York, NY).
P. Crook, N. S. Meck and R. B. Rebak, Paper 07481, Corrosion/2007 (Houston, TX: NACE International, 2007).
R. B. Rebak and P. Crook, “Influence of the Environment on the General Corrosion Rate of Alloy 22 (N06022),” in Proceedings of the 2004 ASME Pressure Vessels and Piping Division Conference, July 25–29 2004, San Diego, CA, Vol. 483, pp. 131–136 (American Society of Mechanical Engineers, 2004: New York, NY).
Haynes International Inc., Corrosion Database, Kokomo, IN 1985–2005.
M. Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions (Houston, TX: NACE International, 1974).
CRC Handbook of Chemistry and Physics, 70th Edition, R. C. Weast Editor-in-chief (CRC Press, Inc., Boca Raton, FL 1989).
http://en.wikipedia.org/wiki/Hydrochloric_acid, http://en.wikipedia.org/wiki/Nitric_acid
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rebak, R.B. Mechanisms of Inhibition of Crevice Corrosion in Alloy 22. MRS Online Proceedings Library 985, 804 (2006). https://doi.org/10.1557/PROC-985-0985-NN08-04
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/PROC-985-0985-NN08-04