Abstract
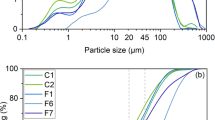
Ash density fractions, separated from a high-Ca fly ash, were leached with 6M HCl at 105°C. The density fractions (<0.79 and >2.85 g.cm−3) selected for leaching have been shown in previous work to consist largely of two different glass types, designated Glass I and Glass II. It was shown that both Glass I and Glass II are leachable to yield solutions containing Al and modifier cations. Acid attack on Glass I appears to involve hydrolysis of Si–O–Al (silalane) bridges along with ion exchange at available non-bridging oxygen sites. Attack on Glass II is more extensive, with both silalane and siloxane bridges being hydrolysed, and ion-exchange occurring at non-bridging oxygens. The products of leaching are aluminosilicates. In the case of Glass I, these remain as pseudomorphic, hollow spheres; whereas, for Glass II, substantial reprecipitation of silica (alumina) gel was found.
Similar content being viewed by others
References
R.T. Hemmings and E.E. Berry, in Fly Ash and Coal Conversion By-products; Characterization. Utilization and Disposal II. edited by G.J. McCarthy, F.P. Glasser and D.M. Roy, Mat. Res. Soc. Symp. Proc Vol. 65 (Materials Research Society, Pittsburgh, 1986) pp. 91–104.
R.T. Hemmings, E.E. Berry, B.J. Cornelius and B.E. Scheetz, in Fly Ash and Coal Conversion By-products: Characterization. Utilization and Disposal III, edited by G.J. McCarthy, F.P. Glasser and D.M. Roy, Mat. Res. Soc. Symp. Proc Vol. 86, (Materials Research Society, Pittsburgh, 1987) pp. 81–98.
R.T. Hemmings and E.E. Berry, this volume.
H. Leitch, H.G. Iverson and J.B. Clemmer, Extraction of Alumina by Leaching Melted Quenched Anorthosite in Sulfuric Acid. USBM, RI 6744, 1966.
R.M. Canon, T.M. Gilliam and J.S. Watson, Evaluation of Potential Processes for Recovery of Metals from Coal Ash. EPRI Report CS-1922, Vols. 1 and 2, 1981.
R.F. Wilder, P.J. Barrett, L.W. Henslee and D. Arpi, Recovery of Metal Oxides from Fly Ash. EPRI Report CS-3544, 3 vols, June 1984.
R.F. Wilder and D.L. Markman, Recovery of Metal Oxides from Fly Ash. Including Ash Beneficiation Product: Product Market Survey Report EPRI Report CS-2422, May 1985.
E.E. Berry, R.T. Hemmings and D.M. Golden, in Fly Ash and Coal Conversion By-products: Characterization. Utilization and Disposal III, edited by G.J. McCarthy, F.P. Glasser and D.M. Roy, Mat. Res. Soc. Symp. Proc Vol. 86 (Materials Research Society, Pittsburgh, 1987) pp. 365–380.
R.T. Hemmings and E.E. Berry, Evaluation of Plastic Filler Applications for Leached Fly Ash, EPRI Report RP 2422–8, May 1985.
L.D. Hulett and A.J. Weinberger, Env. Sci. Technol. 14, 965 (1980); L.D. Hulett, A.J. Weinberger, K.J. Northcutt and N.M. Ferguson, Science 210, 1356 (1980); L.D. Hulett, A.J. Weinberger, N.M. Ferguson, K.J. Northcutt and W.S. Lyon, in Trace Elements and Phase Relations in Flv Ash. EPRI Report EA-1822, May 1981.
The Chemistry of Silica, The Chemistry of Silica. (John Wiley & Sons, New York, 1979), p. 511.
K.J. Murata and W.G. Schlecht, US. Geol. Survey Bull. 950, pp. 25–33, 35–82, (1946).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berry, E.E., Hemmings, R.T. & Cornelius, B.J. Speciation in Size and Density Fractionated Fly Ash III. The influence of HCL Leaching on the Glassy Constituents of a High-Ca Fly Ash. MRS Online Proceedings Library 113, 55–63 (1987). https://doi.org/10.1557/PROC-113-55
Published:
Issue Date:
DOI: https://doi.org/10.1557/PROC-113-55