Abstract
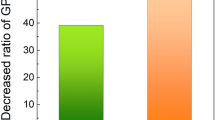
The effect of changes in poly(acrylic acid) (PAA) conformation on removal of Si3N4 film was investigated. PAA was used as a passivation agent by adsorption on an Si3N4 film in shallow-trench isolation chemical–mechanical planarization (STI CMP). Adsorption behavior of PAA on the Si3N4 film and the conformation transition were determined by adsorption isotherms and force measurements using atomic force microscopy (AFM) as a function of ionic strength. AFM results revealed that, as ionic strength increases, the repulsive force between the negatively charged carboxylate groups along the backbone of PAA is reduced due to counterion screening and to the changes of PAA conformation from a stretched to a coiled configuration. At high ionic strength, the coiled conformation of PAA formed a dense passivation layer on the Si3N4 film, which led to suppression of the removal rate of Si3N4 film from 72 to 61 Å/min in the STI CMP process.







Similar content being viewed by others
References
S. Wolf: Deep-Submicron Process Technology Lattice Press Sunset Beach, CA 2002 433
K. Rajiv-Singh R. Bajaj: Advances in chemical–mechanical planarization. MRS Bull. 27, 743 2002
X. Feng, D.C. Sayle, Z.L. Wang, M.S. Paras, B. Santora, A.C. Sutorik, T.X.T. Sayle, Y. Yang, Y. Ding, X. Wang Y-S. Her: Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science 312, 1504 2006
W. Phillip-Carter T.P. Johns: Interfacial reactivity between ceria and silicon dioxide and silicon nitride surfaces. Electrochem. Solid-State Lett. 8, G218 2005
S-K. Kim, U. Paik, S-G. Oh, Y-K. Park, T. Katoh J-G. Park: Effects of the physical characteristics of cerium oxide on plasma-enhanced tetraethylorthosilicate removal rate of chemical mechanical polishing for shallow trench isolation. Jpn. J. Appl. Phys. 42, 1227 2003
H-G. Kang, T. Katoh, S-J. Kim, U. Paik, H-S. Park J-G. Park: Effects of grain size and abrasive size of polycrystalline nano-particle ceria slurry on shallow trench isolation chemical mechanical polishing. Jpn. J. Appl. Phys. 43, L365 2004
J-G. Park, T. Katoh, W-M. Lee, H. Jeon U. Paik: Surfactant effect on oxide-to-nitride removal selectivity of nano-abrasive ceria slurry for chemical mechanical polishing. Jpn. J. Appl. Phys. 42, 5420 2003
T. Katoh, H-G. Kang, U. Paik J-G. Park: Effects of abrasive morphology and surfactant concentration on polishing rate of ceria slurry. Jpn. J. Appl. Phys. 42, 1150 2003
J.P. Kim, J.G. Yeo, U. Paik, Y.G. Jung, J.G. Park V.A. Hackley: Modification of electrokinetic behavior of CeO2 abrasive particles in chemical mechanical polishing for shallow trench isolation. J. Kor. Phys. Soc. 39, S197 2001
H. Nojo, M. Kodera R. Nakata: Slurry engineering for self-stopping, dishing free SiO2-CMP. IEDM Tech. Digest 96, 349 1996
W.G. America S.V. Babu: Slurry additive effect on the suppression of silicon nitride removal during CMP. Electrochem. Solid-State Lett. 7, G327 2004
C-W. Cho, S-K. Kim, U. Paik, J-G. Park W.M. Sigmund: Atomic force microscopy study of the role of molecular weight of poly(acrylic acid) in chemical mechanical planarization for shallow trench isolation. J. Mater. Res. 21, 473 2006
H-G. Kang, T. Katoh, H-S. Park, U. Paik J-G. Park: Effects of abrasive size and surfactant concentration on the non-Prestonian behavior of ceria slurry in shallow trench isolation chemical mechanical polishing. Jpn. J. Appl. Phys. 44, L136 2005
J.R. Kanicky D.O. Shah: Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J. Colloid Interface Sci. 256, 201 2002
K. Ishiduki K. Esumi: The effect of pH on adsorption of poly(acrylic acid) and poly(vinylpyrrolidone) on alumina from their binary mixtures. Langmuir 13, 1587 1997
G.H. Kirby, D.J. Harris, Q. Li J.A. Lewis: Poly(acrylic acid)-poly(ethylene oxide) comb polymer effects on BaTiO3 nanoparticle suspension stability. J. Am. Ceram. Soc. 82, 181 2004
J.A. Lewis: Colloidal processing of ceramics. J. Am. Ceram. Soc. 83, 2341 2000
V.A. Hackley: Colloidal processing of silicon nitride with poly(acrylic acid), I: Adsorption and electrostatic interactions. J. Am. Ceram. Soc. 80, 2315 1997
M.R. Böhmer, O.A. Evers J.M.H.M. Scheutjens: Weak polyelectrolytes between two surfaces: adsorption and stabilization. Macromolecules 23, 2288 1990
C. Wang, K.C. Tam R.D. Jenkins: Dissolution behavior of HASE polymers in the presence of salt. J. Phys. Chem. B 106, 1195 2002
K. Vermöhlen, H. Lewandowski, H-D. Narres M.J. Schwuger: Adsorption of polyelectrolytes onto oxides—The influence of ionic strength, molar mass, and Ca2+ ions. Colloids Surf. A 163, 45 2000
J. Wang P. Somasundaran: Reversible conformational behavior of poly(acrylic acid) LB film with changes in pH, ionic strength and time. Colloids Surf. A 273, 63 2006
R.R. Vedula H.G. Spencer: Adsorption of poly(acrylic acid) on titania (anatase) and zirconia colloids. Colloids Surf. 58, 99 1991
M.J. Benecky, R.W. Wine, C.G. Kolvenbach M.W. Mosesson: Ionic-strength- and pH-dependent conformational states of human plasma fibronectin. Biochemistry 30, 4298 1991
H.G.M. de van Steeg, C.M.A. Stuart, A. De Keizer B.H. Bijsterbosch: Polyelectrolyte adsorption: A subtle balance of forces. Langmuir 8, 2538 1992
M. Colic, G.V. Franks, M.L. Fisher F.F. Lange: Effect of counterion size on short range repulsive forces at high ionic strengths. Langmuir 13, 3129 1997
G.B. Basim B.M. Moudgil: Effect of soft agglomerates on CMP slurry performance. J. Colloid Interface Sci. 256, 137 2002
G.B. Basim, J.J. Adler, U. Mahajan, R.K. Singh B.M. Moudgil: Effect of particle size of chemical mechanical polishing slurries for enhanced polishing with minimal defects. J. Electrochem. Soc. 147, 3523 2000
S.K. Kim, P.W. Yoon, U. Paik, T. Katoh J.G. Park: Influence of physical characteristics of ceria particles on polishing rate of chemical mechanical planarization for shallow trench isolation. Jpn. J. Appl. Phys. 43, 7427 2004
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, YH., Lee, SM., Lee, KJ. et al. Constraints on removal of Si3N4 film with conformation-controlled poly(acrylic acid) in shallow-trench isolation chemical–mechanical planarization (STI CMP). Journal of Materials Research 23, 49–54 (2008). https://doi.org/10.1557/JMR.2008.0031
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2008.0031