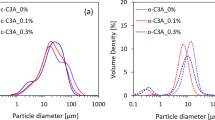
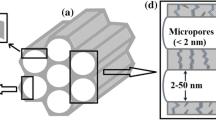
Abstract
The oxidation behavior in air of gas atomized Al–Cu–Fe–Be powders was investigated during isothermal exposures at 750, 800, and 830 °C. Oxidation data obtained at 750 °C for Al–Cu–Fe and Al–Cu–Fe–Cr powders are also presented and used as references. Thermogravimetric analyses showed that Be significantly improved the oxidation resistance of the icosahedral phase at 750 °C. At this temperature the i-phase in Al–Cu–Fe–Be powders was found to be stable even after oxidation for 300 h, while oxidation at and beyond 800 °C led to the formation of a cubic β′-phase. Auger analyses suggested that, in addition to its role on the stability of the icosahedral phase, the presence of Be in the oxide layer provided efficient protection against air oxidation at high temperature.
Similar content being viewed by others
References
C. Janot, Quasicrystals; A Primer, 2nd ed. (Clarendon Press, Oxford, U.K., 1994), p. 22.
U. Koester, W. Liu, H. Liebert, and M. Michel, J. Non-Cryst. Solids 153-154, 446 (1993).
P. Archambault and C. Janot, MRS Bull. 22(11), 48 (1997).
S.S. Kang, J.M. Dubois, and J. von Stebut, J. Mater. Res. 8, 2471 (1993).
J.M. Dubois, P. Plaindoux, E. Belin-Ferre, N. Tamura, and D.J. Sordelet, in 6th International Conference on Quasicrystals, edited by S. Takeuchi and T. Fujiwara (World Scientific, Singapore, 1997), p. 733.
J.M. Dubois, in Quasicrystals: An Introduction to Structure, Physical Properties and Applications, edited by J.B. Suck, M. Schreiber, and P. Haussler (Springer-Verlag, Berlin, Germany, 2002), p. 507.
S.S. Kang and J. Dubois, J. Mat. Res. 10, 1071 (1995).
D. Rouxel, M. Gavatz, P. Pigeat, B. Weber, and P. Plaindoux, in New Horizons in Quasicrystals: Research and Applications, edited by A.I. Goldman, D.J. Sordelet, P.A. Thiel, and J.M. Dubois (World Scientific, Singapore, 1997), p. 173.
D.J. Sordelet, L.A. Gunderman, M.F. Besser, and A.B. Akinc, in New Horizons in Quasicrystals: Research and Applications, edited b y A.I. Goldman, D.J. Sordelet, P.A. Thiel, and J.M. Dubois (World Scientific, Singapore, 1997), p. 296.
C.J. Jenks, P.J. Pinhero, T.E. Bloomer, C.L. Chang, J.W. Anderegg, and P.A. Thiel, in 6th International Conference on Quasicrystals, edited by S. Takeuchi and T. Fujiwara (World Scientific, Singapore, 1998), p. 761.
P.J. Pinhero, D.J. Sordelet, J.W. Anderegg, P. Brunet, J.M. Dubois, and P.A. Thiel, in Quasicrystals, edited by J.M. Dubois, P.A. Thiel, A.P. Tsai, and K. Urban (Mater. Res. Soc. Symp. Proc. 553, Warrendale, PA, 1999), p. 263.
P.J. Pinhero, J.W. Anderegg, D.J. Sordelet, T.A. Lograsso, D.W. Delaney, and P.A. Thiel, J. Mater. Res. 14, 3185 (1999).
P.J. Pinhero, J.W. Anderegg, D.J. Sordelet, M.F. Besser, and P.A. Thiel, Philos. Mag. B 79, 91 (1999).
M. Gil-Gavatz, D. Rouxel, P. Pigeat, B. Weber, and J.M. Dubois, Philos. Mag. A 80, 2083 (2000).
B.I. Wehner and U. Koester, Oxid. Metals 54(5/6), 445 (2000).
J. Jedlinski, Solid State Phenom. 21–22, 335 (1992).
P. Kofstad, in The Role of Active Elements in the Oxidation behavior of High Temperature Metals and Alloys, edited by E. Lang (Kluwer Academic Publishers, Dordrecht, The Netherlands, 1989), p. 367.
V. Demange, J.W. Anderegg, J. Ghanbaja, F. Machizaud, D.J. Sordelet, M. Besser, P.A. Thiel, and J.M. Dubois, Appl. Surf. Sci. 173, 327 (2002).
C. Dong and J.M. Dubois, J. Mater. Sci. 26, 1647 (1991).
Y. Massiani, S. Ait-Yaazza, J.P. Crousier, and J.M. Dubois, J. Non-Cryst. Solids 159, 92 (1993).
G. Bonhomme, M. Lemieux, P. Weisbecker, V.V. Tsukruk, and J.M. Dubois, J. Non-Cryst. Sol. (in press).
J. Kong, C. Zhou, S. Gong, and H. Xu, Surf. Coat. Technol. 165, 281 (2003).
M. Yamasaki and A.P. Tsai, J. Alloys Compd. 342, 473 (2002).
S.M. Lee, B.H. Kim, S.H. Kim, W.T. Kim, and D.H. Kim, Philos. Mag. Lett. 81, 483 (2001).
G.S. Song, E. Fleury, S.M. Lee, W.T. Kim, and D.H. Kim, Mater. Sci. Eng. A 346, 42 (2003).
D.R. Lide, Handbook of Chemistry and Physics, 82nd ed. (CRC Press, Boca Raton, FL, 2001) pp. 4–5.
S.M. Lee, E. Fleury, J.S. Kim, Y.C. Kim, D.H. Kim, W.T. Kim, and H.S. Ahn, in Quasicrystals, edited by A.P. Tsai, E. Belin-Ferre, P. Thiel, and K. Urban (Mater. Res. Soc. Symp. Proc. 643, Warrendale, PA, 2001), K 15.2.1.–15.2.12.
R.A. Swalin, Thermodynamics of Solids (John Wiley & Sons, Singapore, 1991), p. 88.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fleury, E., Kim, J.S., Kim, D.H. et al. High-temperature oxidation of Al–Cu–Fe–Be quasicrystalline powders. Journal of Materials Research 18, 1837–1841 (2003). https://doi.org/10.1557/JMR.2003.0256
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2003.0256