Abstract
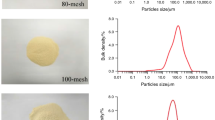
The high-temperature combustion mechanism of the BaO2–TiO2–organic compound system [urea, CO(NH2)2; hexamethylenetetramine, C6H12N4] was investigated by the microthermocouple technique. The adiabatic combustion temperatures (Tad) and equilibrium compositions of the final products were calculated by the computer program THERMO. The distribution of the temperature T(x), rate of heat generation Φ(x), and degree of conversion η(x) in the combustion wave were determined. The relative sizes of the combustion zones, the leading stage of the reaction, the kinetic law of components interaction, and parameters (activation energy, pre-exponential factor, braking parameter) were then calculated. Through this method, the optimal conditions for tetragonal BaTiO3 powder synthesis with spherical form and particles size of 2–5 μm were found.
Similar content being viewed by others
References
A. Dias, V.T.L. Buono, V.S.T. Ciminelli, and R.L. Moreira, J. Eur. Ceram. Soc. 19, 1027 (1999).
T. Hyashi, H. Shinozaki, and K. Sasaki, J. Eur. Ceram. Soc. 19, 1011 (1999).
A. Rae, M. Chu, and V. Ganine, Ceramic Transactions 100, 1 (1999).
P.K. Dutta, R. Asiaie, S.A. Akbar, and W. Zhu, Chem. Mater. 6, 1542 (1994).
J.H. Lee, T.S. Kim, C.W. Won, and H.S. Kim, J. Mater. Sci. 35, 4271 (2000).
D. Hennings, M. Klee, and R. Waser, Adv. Mater. 3, 334 (1991).
M. Okiwa and K. Tada, Appl. Phys. Lett. 29, 491 (1976).
K.W. Kirby, Mater. Res. Bull. 23, 881 (1988).
A.G. Merzhanov, J. Mater. Proc. Tech. 56, 222 (1996).
Z.A. Munir and U. Anselmi-Tamburini, Mater. Sci. Reports 69, 277 (1989).
A.G. Merzhanov, Y. Popova, and D.V. Semiletova, Problemy Teckhnologicheskogo Goreniya. Chernogolovka 2, 15 (1981).
A.Y. Komarov and I.P. Parkin, Polyhedron 15, 1349 (1996).
T.V. Anuradha, S. Ranganathan, T. Mimani, and K.C. Patil, Scripta Mater. 44, 2237 (2001).
H.H. Nersisyan, J.H. Lee, and C.W. Won, J. Mater. Res. 17, 2859 (2002).
A.A. Shiryaev, Int. J. SHS 4, 351 (1995).
A.A. Zenin, A.G. Merzhanov, and H.H. Nersisyan, Dokl. Akad. Nauk USSR 250, 880 (1980).
CRC Handbook of Chemistry and Physics, edited by C.W. Robert (CRC Press, Boca Raton, FL, 1987).
A.G. Merzhanov, Russ. Chem. Bull. 46, 1 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, J.H., Nersisyan, H.H., Simkins, M.L. et al. Mechanism of combustion and phase formation in the BaO2–TiO2–organic compound system. Journal of Materials Research 18, 1926–1933 (2003). https://doi.org/10.1557/JMR.2003.0254
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2003.0254