Abstract
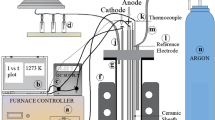
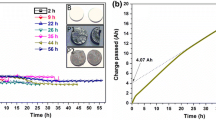
The method of electro-deoxidation was applied to reduce solid Nb2O5 directly to niobium metal, in the form of sponge, in the eutectic CaCl2–NaCl and BaCl2–NaCl systems, respectively. The direct electrochemical reduction of solid Nb2O5 was achieved by electrolysis in the chloride melts at temperatures between 850 and 950 °C and at a controlled potential of 3.1 V, well below the decomposition potentials of the melts used. The obtained results demonstrated that the method is applicable for preparing niobium sponges directly from solid Nb2O5 under the present experimental conditions. The niobium sponge prepared was proven to possess as remarkable superconducting properties. The parameters affecting the rate and the extent of electro-deoxidation were investigated experimentally, including initial particle sizes of the starting Nb2O5 powders, compaction pressure, sintering temperature and times, electrolysis temperature and duration, and electrolyte systems. The mechanism by which the Nb2O5 pellets were electro-deoxidized to the niobium sponges was also discussed on the basis of the present experimental observations.
Similar content being viewed by others
References
O.R. Suzuki, M. Aizawa, and K. Ono, J. Alloys Compd. 288, 173 (1999).
H. Padamsee, J. Less-Common Met. 139, 167 (1988).
Handbook of Applied Superconductivity, edited by B. Seeber (Institute of Physics Publishing, London, U.K., 1998), pp. 1165–1734.
C. Laverick, J. Less-Common Met. 139, 107 (1988).
E. Olzi and F.C. Matacotta, J. Less-Common Met. 139, 123 (1988).
N.P. Lyakishev, N.A. Tulin, and Yu. L. Pilner, Niobium in Steel and Alloys (Companhia Brasileira de Metalurgia e Mineracao-CBMM, Soa Paulo, Brazil, 1984).
H.A. Wilhelm, F.A. Schmidt, and T.G. Ellis, J. Met. 1303 (Dec 1966).
G.R. Kamat and C.K. Gupta, Metall. Trans. 2, 2817 (1971).
O.N. Carlson, in Encyclopedia of Materials Science and Engineering, edited by M.B. Bever (Pergamon Press, New York, 1986), p. 3205.
D.J. Fray, T.W. Farthing, and Z. Chen, Inter. Patent No. PCT/GB99/ 01781 (first filing date: 05 June 1998).
G.Z. Chen, D.J. Fray, and T.W. Farthing, Nature (London) 407, 361 (2000).
G.Z. Chen and D.J. Fray, in Progress in Molten Salt Chemistry, edited by R.W. Berg, H.A. Hjuler (Elsevier, Paris, France, 2000), p. 157.
G.Z. Chen and D.J. Fray, Light Met. 1147 (2001).
D.J. Fray and G.Z. Chen, in Proceedings of the 4th International Conference on Materials Engineering for Resources (The Society of Materials Engineering for Resources of Japan, Akita, Japan, 2001), p. 1.
D.J. Fray, J. Met. 26 (Oct 2001).
B.A. Glowacki, X.Y. Yan, D.J. Fray, G. Chen, M. Majoros, Y. Shi, Physica C (in press).
X.Y. Yan and D.J. Fray, Light Met. 5 (2002).
X.Y. Yan and D.J. Fray, Metall. Mater. Trans. B (in press).
X.Y. Yan and D.J. Fray (submitted for publication).
H.S.C. Outokumpu, Chemistry for Windows, version 4.0 (Outokumpu Research Oy Information Service, Pori, Finland, 1999).
Y.K. Rao, Stoichiometry and Thermodynamics of Metallurgical Processes (Cambridge University Press, London, U.K., 1985), p. 883.
T.H. Okabe, I. Park, K.T. Jacob, and Y. Waseda, J. Alloys Compd. 288, 200 (1999).
I. Park, T.H. Okabe, Y. Waseda, H.S. Yu, and O.Y. Lee, Mater. Trans. 42, 850 (2001).
R. Roberge, in Superconductor Materials Science, edited by S. Foner and B.B. Schwartz (Plenum Press, New York, 1981), p. 442.
P. Chartrand and A.D. Pelton, Metall. Mater. Trans. A 32A, 1361 (2001).
P. Chartrand and A.D. Pelton, Can. Met. Q. 40, 13 (2000).
E.M. Levin and H.F. Mc Murdie, Phase Diagrams for Ceramists-Supplement (The American Ceramic Society, Westerville, OH, 1975).
S. Boghosian, A.A. Godo, H. Mediaas, W. Ravlo, and T. Ostvold, Acta Chem. Scand. 45, 145 (1991).
J. Halbritter, IEEE Trans. Magn. MAG–21, 858 (1985).
J. Halbritter, J. Less-Common Met. 139, 133 (1988).
R. Kirchheim, Acta Metall. 27, 869 (1979).
A.J. Bard and L.R. Faulkner, Electrochemical Methods, 2nd ed. (John Wiley & Sons, New York, 2001), pp. 423–425.
P. Delahay, New Instrumental Methods in Electrochemistry (Interscience Publishers, New York, 1954), pp. 218–219, 283–284.
J. Szekely, J.W. Evans, and H.Y. Sohn, Gas-Solid Reactions (Academic Press, New York, 1976), pp. 73–78.
J.K. Hulm and B.T. Matthias, in Superconductor Materials Science, edited by S. Foner, B.B. Schwartz (Plenum Press, New York, 1981), p. 28.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yan, X.Y., Fray, D.J. Using electro-deoxidation to synthesize niobium sponge from solid Nb2O5 in alkali–alkaline-earth metal chloride melts. Journal of Materials Research 18, 346–356 (2003). https://doi.org/10.1557/JMR.2003.0045
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2003.0045