Abstract
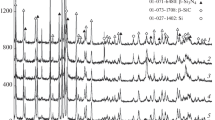
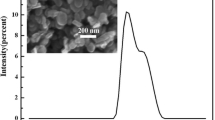
A combustion synthesis (SHS) process developed in our previous study for the synthesis of Si3N4 has been tested for the synthesis of boron nitride (BN) powder. Boron and NaN3 powders were used as the reactants. In addition to ammonium halides, KHF2, FeCl3, NaNH2, or a mixture of 50 mol% FeCl3 and 50 mol% NaNH2 was added to the reactants to examine their catalytic effect. These powders were mixed and pressed into a cylindrical compact. The compact was wrapped up with an igniting agent (i.e., Mg + Fe3O4) and the synthesis reaction was triggered by the combustion of the igniting agent. It was found that only those reagents containing both halogen and hydrogen can exert effectively the catalytic effect. The BN powder as-synthesized is mostly in the form of agglomerated fine particles (0.1–1 μm in diameter) and is hexagonal in crystalline structure. Effects of various experimental parameters on the product yield were investigated. A possible reaction mechanism was proposed, which explains the effects of the experimental parameters on the synthesis reaction.
Similar content being viewed by others
References
V. A. Lavrenko and A. F. Alexeev, Ceram. Int. 12, 25 (1986).
V. Laurence, D. Gérard, and J. Etourneau, Mater. Sci. Eng. B10, 149 (1991).
M. Z. Karim, D. C. Cameron, and M. S. J. Hashmi, Mater. Des. 13, 207 (1992).
A. G. Merzhanov and I. P. Borovinskaya, Combust. Sci. Technol. 10, 195 (1975).
Z. A. Munir, Ceram. Bull. 67, 342 (1988).
J. F. Crider, Ceram. Eng. Sci. Proc. 3, 519 (1982).
W. C. Lee, C. L. Tu, C. Y. Weng, and S. L. Chung, J. Mater. Res. 10, 774 (1995).
W. C. Lee and S. L. Chung, J. Mater. Res. 12, 805 (1997).
A. G. Massey, Adv. Inorg. Chem. Radiochem. 10, 1 (1967).
M. W. Chase, Jr., C. A. Davies, J. R. Downey, Jr., D. J. Frurip, R. A. Donald, and A. N. Syverud, JANAF Thermochemical Tables, 3rd Ed. (ACS and AIP for NBS, New York, 1986).
H. O. Pierson, J. Compos. Mater. 9, 228 (1975).
T. Matsuda, N. Uno, H. Nakae, and T. Hirai, J. Mater. Sci. 21, 649 (1986).
V. Pavlović, H-R. Kötter, and C. Meixner, J. Mater. Res. 6, 2393 (1991).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, CC., Chung, SL. Combustion synthesis of boron nitride powder. Journal of Materials Research 13, 680–686 (1998). https://doi.org/10.1557/JMR.1998.0085
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.1998.0085