Abstract
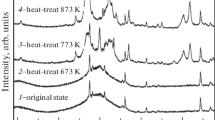
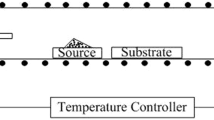
In2O3–SnO2 films were produced by thermal decomposition of a deposit which was dip coated on borosilicate glass substrates from an acetylacetone solution of indium and tin acetoacetonate. Thermal analysis showed complete pyrolysis of the organics by 400 °C. The thermal decomposition reaction generated acetylacetone gas and was found to be first order with an activation energy of 13.6 Kcal/mole. Differences in thermal decomposition between the film and bulk materials were noted. As measured by differential scanning calorimetry using a 40 °C/min temperature ramp, the glass transition temperature of the deposited oxide film was found to be ∼462 °C, and the film crystallization temperature was found to be ∼518 °C. For film fabrication, thermal decomposition of the films was performed at 500 °C in air for 1 h followed by reduction for various times at 500 °C in a reducing atmosphere. Crystalline films resulted for these conditions. A resistivity of ∼1.01 × 10−3 Ω · cm, at 8 wt. % tin oxide with a transparency of ∼95% at 400 nm, has been achieved for a 273 nm thick film.
Similar content being viewed by others
References
T. Maruyama and A. Kojima, Jpn. J. Appl. Phys. 27 (10), L1829–1831 (1988).
A.L. Dawar and J.C. Joshi, J. Mater. Sci. 19, 1–23 (1984).
N. J. Arfsten, R. Kaufman, and H. Dislich, in Ultrastructure Processing of Advanced Ceramics, edited by J. D. Mackenzie and D.R. Ulrich (John Wiley, New York, 1984), pp. 189–196.
N. J. Arfsten, R. Kaufman, and H. Dislich, German Patent DE 3300589, July 12, 1984.
I. Hamberg and C.G. Granqvist, J. Appl. Phys. 60 (11), R123–R159 (1986).
G. Frank, H. Kösflin, and A. Rabenau, Phys. Status Solidi (A) 52, 231–238 (1979).
J. C. C. Fan and J. B. Goodenough, J. Appl. Phys. 48 (8), 3524–3531 (1977).
Desag, Grünenplan, Germany (AF-45).
Omicron Spectrometer, Kevex Instruments, San Carlos, CA 94070.
SFM-BD2-210, Park Scientific Instruments, Mountain View, CA 94043.
Fluka, Buchs, Switzerland (technical grade).
Fluka, Buchs, Switzerland (reagent grade).
Nicolet 510 FTIR spectrometer, Nicolet Analytical, Madison, WI.
Carbagas, Lausanne, Switzerland (technical grade).
Mettler TG 50, Mettler, Zurich, Switzerland.
Mettler DSC 30, Mettler, Zurich, Switzerland.
Chemical Data System Pyroprobe 200, CDS, Oxford, PA.
Varian 3400, Varian Ass., Sunnyvale, CA.
Finnigan-MAT Ion Trap Mass Spectrometer ITMS, Finnigan-MAT, San Jose, CA.
F. P. Scanlan and R. Houriet, J. Trace Microprobe Technol. 9, 177–199 (1991).
PHI 5500 Perkin-Elmer, Norwalk, CT 00856.
A-DIDA 3000(Atomika) Perkin-Elmer, Norwalk, CT 00856.
L. J. van der Pauw, Philips Res. Rep. 13 (1), 1–9 (1958).
S.M. Sze, Physics of Semiconductor Devices, 2nd Edition (John Wiley & Sons, New York, 1981), pp. 31–33.
Perkin-Elmer Lambda 6, Norwalk, CT 00856.
J. D. Roberts and M. C. Caserio, Basic Principles of Organic Chemistry (W. A. Benjamin, Inc., New York, 1965), p. 498.
This peak at 346 nm is similar to that observed for A1(C5H7O2)2.
A.L. Allred and D.W. Thompson, Inorg. Chem. 7, 1196–1201 (1968).
R.W. Jones and R.C. Fay, Inorg. Chem. 12, 2599–2606 (1973).
J.W. Faller and A. Davidson, Inorg. Chem. 6, 182–184 (1967).
D.W. Thompson, J.F. Lefelhoxz, and K.S. Wong, Inorg. Chem. 11, 1139–1141 (1972).
N. Inagaki and J. Ohkubo, J. Appl. Polym. Sci. 43 (4), 793–800 (1991).
B.V. Deryagin, DAN USSR. 39, 11 (1943); reviewed in B.M. Deryagin and S.M. Levi, The Focal Press, London (1964).
N. B. Morozova, V. N. Mit’kin, and I. K. Igumenov, Russ. J. Inorg. Chem. 33 (10), 1459–1464 (1988).
P. Sharpe and D.E. Richardson, J.Am. Chem. Soc. 113, 8339–8340 (1991).
Ta2O5/Ta certified reference material, CRM 261 R, BCR Brussels.
J.L. Vossen, RCA Rev. 32, 289–296 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gallagher, D., Scanlan, F., Houriet, R. et al. Indium-tin oxide thin films by metal-organic decomposition. Journal of Materials Research 8, 3135–3144 (1993). https://doi.org/10.1557/JMR.1993.3135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.1993.3135