Abstract
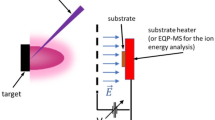
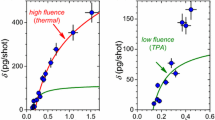
A versatile, repetitively pulsed source of translationally fast, reactive molecules is described that is suitable for materials processing experiments. The pulsed beams are generated by excimer laser vaporization of cryogenic molecular films that are continuously condensed on transparent substrates. The generation of fast, energy variable pulsed molecular sources of Cl2 and NO is demonstrated. The most probable translational energies of Cl2 and NO molecules can be reproducibly varied monotonically by adjusting the laser fluence or film thickness. Here, the most probable translational energy is quoted as the energy corresponding to the maximum of the time-of-flight trace. Using laser fluences of 2–25 mJ cm−2 from a 193 nm excimer laser, the most probable translational energies of Cl2 are 0.4–2 eV. Significant fractions of molecules with translational energies greater than 3 eV are observed at the leading edges of the distributions. Very similar results are obtained by vaporizing Cl2 with 248 and 351 nm radiation. Pulses of translationally fast NO molecules are generated in a similar manner; most probable energies from 0.1–0.4 eV, with the fastest molecules up to 0.8 eV, are obtained using laser fluences of 1–11 mJ cm−2 at 193 nm. Approximately 1013−1014 molecules per cm2 of the film are vaporized per laser pulse, depending on film thickness and laser fluence.
Similar content being viewed by others
References
Dry Etching for Microelectronics, edited by R. A. Powell (Elsevier, Amsterdam, 1984).
Materials Modification and Growth Using Ion Beams, edited by U. J. Gibson, A. E. White, and P. P. Pronko (Materials Research Society, Pittsburgh, PA, 1987).
J. W. Coburn and H. F. Winters, J. Vac. Sci. Technol. 16, 391 (1979).
J. W. Coburn and H. F. Winters, J. Appl. Phys. 50, 3189 (1979).
H. F. Winters, J. Vac. Sci. Technol. A 3, 700 (1985).
H. F. Winters, J. Vac. Sci. Technol. B 3, 9 (1985).
T. Mizutani, C. J. Dale, W. K. Chu, and T. M. Mayer, Nucl. In-strum. Methods B 7/8, 825 (1985).
D. J. Oostra, A. Haring, and A. E. deVries, J. Vac. Sci. Technol. B 4, 1278 (1986).
D. J. Oostra, A. Haring, A. E. deVries, F. H. M. Sanders, and G. N. A. Van Veen, Nucl. Instrum. Methods B 13, 556 (1986).
R. A. Zuhr, G. D. Alton, B. R. Appleton, N. Herbots, T. S. Noggle, and S. J. Pennycock, in Ref. 2, p. 243.
B. R. Appleton, S. J. Pennycock, R. A. Zuhr, N. Herbots, and T. S. Noggle, Nucl. Instrum. Methods B 19/20, 975 (1987).
R. A. Zuhr, B. R. Appleton, N. Herbots, B. G. Larson, T. S. Noggle, and S. J. Pennycock, J. Vac. Sci. Technol. A 5, 2135 (1987).
B. W. Dodson, Phys. Rev. B 36, 1068 (1987).
B. J. Garrison, M T. Mitchell, and D. W. Brenner, Chem. Phys. Lett. 146, 553 (1988).
B. W. Dodson and P. A. Taylor, J. Mater. Res. 2, 805 (1987).
G. W. Flynn and R. E. Weston, Jr., Ann. Rev. Phys. Chem. 37, 551 (1986).
N. Abauf, J. B. Anderson, R. P. Andres, J. B. Fenn, and D. G. H. Marsden, Science 155, 997 (1967).
J. F. Friichtenicht, Rev. Sci. Instrum. 45, 51 (1974).
S. P. Tang, N. G. Utterback, and J. F. Friichtenicht, J. Chem. Phys. 64, 3833 (1976).
B. G. Wicke, S. P. Tang, and J. F. Friichtenicht, Chem. Phys. Lett. 53, 304 (1977).
B. G. Wicke, J. Chem. Phys. 78, 6036 (1983).
K. Domen and T. J. Chuang, Phys. Rev. Lett. 59, 1484 (1987).
I. Harrison, J. C. Polanyi, and P. A. Young, J. Chem. Phys. 89, 1498 (1988).
N. Nishi, H. Shinohara, and T. Okuyama, J. Chem. Phys. 80, 3898 (1984).
D. E. Brinza, D. R. Coulter, R. H. Liang, A. Gupta, in Proceedings of the NASA Workshop on Atomic Oxygen Effects, edited by D. E. Brinza (JPL, Pasadena, CA, 1987).
D. Bäuerle, Chemical Processing with Lasers, Springer Series in Materials Science (Springer, Berlin, 1986), Vol. 1.
R. Srinivasan, Science 234, 559 (1986); E. Sutcliffe and R. Srinivasan, J. Appl. Phys. 60, 3315 (1986).
J. E. Rothenberg and R. Kelly, Nucl. Instrum. Methods B 1, 291 (1984).
R. W. Dreyfus, R. Kelly, and R. E. Walkup, Appl. Phys. Lett. 49, 1478 (1986); M. Eyett and D. Bäuerle, Appl. Phys. Lett. 51, 2054 (1988).
W. L. Brown, L. J. Lanzerotti, K. J. Marcantonio, R. E. Johnson, and C. T. Reimann, Nucl. Instrum. Methods B 14, 392 (1986).
L. M. Cousins and S. R. Leone (in preparation).
H. Okabe, Photochemistry of Small Molecules (Wiley, New York, 1978), p. 185.
M. E. Fajardo, V. A. Apkarian, A. Maustakas, H. Krueyer, and E. Weitz, J. Phys. Chem. 92, 357 (1988).
M. Suzuki, T. Yokoyama, and M. Ito, J. Chem. Phys. 50, 3392 (1969).
T. Herzog and G. M. Schwab, Z. Phys. Chem. 66, 190 (1969).
E. I. Yakovenko, G. B. Serteev, and G. P. Kalinina, Dauk. Akad. Nauk. SSSR 173, 626 (1966).
R. W. Dreyfus, R. Kelly, and R. E. Walkup, Nucl. Instrum. Methods B 23, 557 (1987).
J. P. Cowin, D. J. Auerbach, C. Becker, and L. Wharton, Surf. Sci. 78, 545 (1978).
N. G. Utterback, S. P. Tang, and J. F. Friichtenicht, Phys. Fluids 19, 900 (1976).
F. Cottet and J. P. Romain, Phys. Rev. A 25, 576 (1982).
P. E. Dyer and R. Srinivasan, Appl. Phys. Lett. 48, 445 (1986).
J. C. Carls and J. R. Brock, Opt. Lett. 13, 273 (1988).
H. Schoeffman, H. Schmidt-Kloiber, and E. Reichel, J. Appl. Phys. 63, 46 (1987).
R. Srinivasan and A. P. Ghosh, Chem. Phys. Lett. 143, 546 (1988).
I. S. Bitensky and E. S. Parilis, Nucl. Instrum. Methods B 21, 26 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cousins, L.M., Leone, S.R. Production of 0.1–3 eV reactive molecules by laser vaporization of condensed molecular films: A potential source for beam-surface interactions. Journal of Materials Research 3, 1158–1168 (1988). https://doi.org/10.1557/JMR.1988.1158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.1988.1158