Abstract
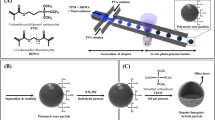
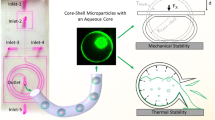
This paper presents a new route to the synthesis of uniform and size-controlled inorganic/organic composite microparticles by means of microreaction technology. Au-nanoparticles in the range of 3 to 14 nm are synthesized by reduction of tetrachloroauric acid, while ZnO-nanoparticles (200–2000 nm) are synthesized in a continuous-flow twostep process using microtube arrangements for microsegmented flow. Both inorganic nanoparticles have a wellcontrolled size and narrow size distribution. Upon surface modification, the nanoparticles are then mixed on one hand with an acrylate-based monomer and, on the other hand, with an aqueous solution of acrylamide. Both solutions were then emulsified into uniform core-shell droplets by means of a capillary-based microfluidic device. Droplet’s shell was hardened through UV-induced polymerization, whereas the core led to a hydrogel upon thermal-induced polymerization. Core-shell polymer microparticles (200–300 μm) with inorganic nanoparticles selectively incorporated into the core and the shell are thus obtained as proven by extensive morphological characterizations using electronic and optical microscopies.
Article PDF
Similar content being viewed by others
References
Chang, Z.; Serra, C. A.; Bouquey, M.; Kraus, I.; Li, S.; Köhler, J. M. Nanotechnology, 2010, 21, 015605.
Cottam, B. F.; Krishnadasan, S.; DeMello, A. J.; DeMello, J. C.; Shaffer, M. S. P. Lab. Chip. 2007 167–169
Donnet, M.; Jongen, N.; Lemaitre, J.; Bowen, P. J. Mat Sci. Lett. 2000, 19, 749–750.
Guillemet-Fritsch, S.; Aoun-Habbache, S.; Sarrias, J.; Rousset, A.; Jongen, N..; Donnet, M.; Bowen, P.; Lemaitre J. Solid State Ionics 2004, 171, 135–140.
Winterton, J. D.; Myers, D. R.; Lippmann, J. M.; Pisano, A. P.; Doyle, F. M. J. Nanopart. Res. 2008, 10, 893–905.
Wagner, J.; Köhler, J. M. Nano Lett. 2005, 5, 685–691
Weng, C.-H.; Huang, C.-C.; Yeh, C.-S.; Lei, H-Y.; Lee, G.-B. J. Micromech. Microengn. 2008, 18, 035019.
Cabeza, V. S.; Kuhn, S.; Kulkarni, A. A.; Jensen, K. F. Langmuir 2012, 28, 7007–7013.
Knauer, A.; Thete, A.; Li, S.; Romanus, H.; Csaki, A.; Fritzsche, W.; Köhler, J. M. Chem. Eng. J. 2011, 116, 1164–1169.
Knauer, A.; Csaki, A.; Möller, F.; Hühn, C.; Fritzsche, W.; Köhler, J. M. J. Phys. Chem. 2012, 116, 9251–9258.
Gross, G. A.; Hamann, C.; Günther, M.; Köhler, J. M. Chem. Eng. Technol. 2007, 30, 341–346.
Köhler, J. M.; Kraus, I.; Faerber, J.; Serra, Ch. J. Mat. Sci. 2013, 48, 2158–2166.
Köhler, J. M.; März, A.; Popp, J.; Knauer, A.; Kraus, I.; Faerber, J.; Serra, C. Anal. Chem. 2013, 85, 313–318.
Link, S.; Wang, Z. L.; El-Sayed, M. A. J. Phys. Chem. 1999, B103, 3529–3533.
Moskovits, M.; Smova-Sloufova, I.; Vlckovâ, B. J. Chem. Phys. 2002, 116, 10435–10446.
Rodriguez-Gonzâlez, B.; Burrows, A.; Wanatabe, M.; Kiely, C. J.; Liz Marzan, L. M. J. Mat. Chem. 2005, 15, 1755–1759.
Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A. P. Lab Chip 2008, 8, 198–220.
Aoki, N.; Mae, K. Chem. Eng. J. 2006, 118, 189–197.
Köhler, J. M.; Held, M.; Hübner, U.; Wagner, J. Chem. Eng. Tech. 2007, 30, 347–354.
Li, S.; Günther, P. M.; Köhler, J. M. J. Chem. Eng. 2009, 42, 338–345.
Serra, C.; Chang, Z. Chem. Eng. Tech. 2008, 31, 1099–1115.
Serra, C.; Berton, N.; Bouquey, M.; Prat, L.; Hadziioannou, G. Langmuir 2007, 23, 7745–7750.
Sawyer, L. C.; Grubb, D. T. Polymer microscopy; Chapman and Hall, London, UK, 1996.
Jönsson, J.-E.; Karlsson, O. J.; Hassender, H.; Törnell, B. Eur. Poly. J. 2007, 43, 1322–1332.
Kirner, T.; Albert, J.; Günther, M.; Mayer, G.; Reinhäckel, K.; Köhler, J. M. Chem. Eng. J. 2004, 101, 65–74.
Köhler, J. M.; Wagner, J.; Albert, J. J. Mat. Chem. 2005, 15, 1924–1930.
Polte, J.; Ahner, T.; Delissen, F.; et al. J. Am. Chem. Soc. 2010, 132, 1296–1301
Li, S.; Meierott, S.; Köhler, J. M. Chem. Eng. J. 2010, 165, 958–965.
Köhler, J. M.; Li, S.; Knauer, A. Chem. Eng. Technol. 2013, 36, 887–899.
Chang, Z.; Serra, C.; Bouquey, M.; Prat, L.; Hadziioannou, G. Lab. Chip. 2009, 9, 3007–3011.
Yobas, L.; Martens, S.; Ong, W. L.; Ranganathan, N. Lab. Chip. 2006, 6, 1073–1079.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kraus, I., Li, S., Knauer, A. et al. Continuous-Microflow Synthesis and Morphological Characterization of Multiscale Composite Materials Based on Polymer Microparticles and Inorganic Nanoparticles. J Flow Chem 4, 72–78 (2014). https://doi.org/10.1556/JFC-D-13-00029
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/JFC-D-13-00029