Abstract
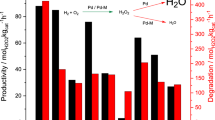
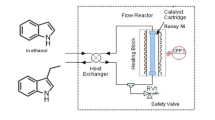
A process comprising a continuous-flow hydrogenation reaction integrated with selective water-organic solvent biphasic extraction using CO2 as molecular switch to control partitioning was devised for the synthesis of arylpiperidines from arylpyridines. The selective hydrogenation of 4-phenylpyridine using heterogeneous carbon-supported metal catalysts was chosen as model reaction. A design-of-experiment approach was used for the identification of suitable reaction conditions under continuous-flow operation. A maximum selectivity for 4-phenylpiperidine of 96% was achieved at 87% conversion suppressing the deep hydrogenation to 4-cyclohexylpiperidine almost completely (≤5%). The higher basicity of piperidines over pyridines was exploited for selective and reversible protonation of the product upon pressurization with CO2 separating it quantitatively from the remaining starting material in a water-EtOAc biphasic system. This concept enabled a fully integrated and a salt-free synthetic process using a standard Pd/C catalyst for the hydrogenation coupled with the CO2-triggered isolation of the desired product 4-phenylpiperidine in 81% yield and 98% purity.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Buffat, M. G. P. Tetrahedron 2004, 60, 1701–1729.
Glorius, F. Org. Biomol. Chem. 2005, 3, 4171–4175.
Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893–930.
Hamilton, T. S.; Adams, R. J. Am. Chem. Soc. 1928, 50, 2260–2263.
Freifelder, M. J. Org. Chem. 1962, 27, 4046.
Freifelder, M. J. Org. Chem. 1963, 28, 602–603.
Freifelder, M. J. Org. Chem. 1964, 29, 2895–2898.
Freifelder, M.; Robinson, R. M.; Stone, G. R. J. Org. Chem. 1962, 27, 284–286.
Freifelder, M.; Stone, G. R. J. Org. Chem. 1961, 26, 3805–3808.
Freifelder, M.; Wright, H. B. J. Med. Chem. 1964, 7, 664–665.
Ryashentseva, M. A.; Minachev, K. M.; Dorogov, V. V.; Prostakov, N. S. Chem. Heterocycl. Compd. 1972, 8, 82–84.
Ryashentseva, M. A.; Prostakov, N. S. Chem. Heterocycl. Compd. 1992, 28, 1229–1235.
Kim D.-I., Deutsch, H. M.; Ye, X.; Schweri, M. M. J. Med. Chem. 2007, 50, 2718–2731.
Díaz, J. L.; Fernández-Forner, D.; Bach, J.; Lavilla, R. Synth. Commun. 2008, 38, 2799–2813.
Mach, U. R.; Hackling, A. E.; Perachon, S.; Ferry, S.; Wermuth, C. G.; Schwartz, J.-C.; Sokoloff, P.; Stark, H. ChemBioChem 2004, 5, 508–518.
Overberger, C. G.; Herin, L. P. J. Org. Chem. 1962, 27, 417–422.
Subramanyam, C.; Chattarjee, S.; Mallamo, J. P. Tetrahedron Lett. 1996, 37, 459–462.
Maxted, E. B.; Walker A. G. J. Chem. Soc. 1948, 1093–1097.
Irfan, M.; Petricci, E.; Glasnov, T. N.; Taddei, M.; Kappe, C. O. Eur. J. Org. Chem. 2009, 1327–1334.
Irfan, M.; Glasnov, T. N.; Kappe, C. O. ChemSusChem 2011, 4, 300–316.
Prat, D.; Hayler, J.; Wells, A. Green Chem. 2014, 16, 4546–4551.
Sheldon, R. A. Green Chem. 2007, 9, 1273–1283.
Sheldon, R. A. Green Chem. 2017, 19, 18–43.
Gutmann, B.; Cantillo, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2015, 54, 6688–6728.
Jiménez-González, C.; Poechlauer, P.; Broxterman, Q. B.; Yang B.-S.; am Ende, D.; Baird, J.; Bertsch, C.; Hannah, R. E.; Dell’Orco, P.; Noorman, H.; Yee, S.; Reintjens, R.; Wells, A.; Massonneau, V.; Manley, J. Org. Process Res. Dev. 2011, 15, 900–911.
Anastas, P.; Han, B.; Leitner, W.; Poliakoff, M. Green Chem. 2016, 18, 12–13.
Pollet, P.; Davey, E. A.; Urena-Benavides, E. E.; Eckert, C. A.; Liotta, C. L. Green Chem. 2014, 16, 1034–1055.
Baxendale, I. R.; Braatz, R. D.; Hodnett, B. K.; Jensen, K. F.; Johnson, M. D.; Sharratt, P.; Sherlock, Florence, A. J. J. Pharm. Sci. 2015, 104, 781–791.
Leitner, W.; Jessop, P. G. Handbook of Green Chemistry, Vol. 4; Supercritical Solvents; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2010.
Franciò, G.; Hintermair, U.; Leitner, W. Philos. Trans. R. Soc. London, Ser. A 2015, 373, DOI: 10.1098/rsta.2015.0005.
Jessop, P. G.; Mercer, S. M.; Heldebrant, D. J. Energy Environ. Sci. 2012, 5, 7240–7253.
Jessop, P. G. Aldrichim Acta 2015, 48, 18–21.
Enick, R. M.; Beckman, E. J.; Shi, C.; Xu, J.; Chordia, L. Energy Fuels 2001, 15, 256–262.
Rieger, W. H.; Springman, L. A. REILLY TAR & CHEM CORP, Purification of piperidines, 1959-01-13, US2868793, 1959.
Xiong, D.; Li, Z.; Wang, H.; Wang, J. Green Chem. 2013, 15, 1941–1948.
Franciò, G.; Leitner, W.; de Wispelaere, I. M.; Rheinisch Westfälische Technische Hochschule Aachen; Verfahren zur Herstellung von Aminen; 2014-11-27, DE102013105317A1, 2014.
Lide, D. R. CRC Handbook of Chemistry and Physics, 85th edn.; CRC Press: Boca Raton, 2004.
Prat, D.; Hayler, J.; Wells, A. Green Chem. 2014, 16, 4546–4551.
Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; Hosek, P. Org. Process Res. Dev. 2013, 17, 1517–1525.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barwinski, B., Migowski, P., Gallou, F. et al. Continuous-Flow Hydrogenation of 4-Phenylpyridine to 4-Phenylpiperidine with Integrated Product Isolation Using a CO2 Switchable System. J Flow Chem 7, 41–45 (2017). https://doi.org/10.1556/1846.2017.00003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/1846.2017.00003