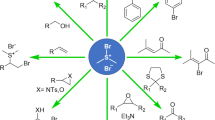
Abstract
Continuous-flow generation of α-diazosulfoxides results in a two- to three-fold increase in yields and decreased reaction times compared to standard batch synthesis methods. These high yielding reactions are enabled by flowing through a bed of polystyrene-supported base (PS-DBU or PS-NMe2) with highly controlled residence times. This engineered solution allows the α-diazosulfoxides to be rapidly synthesized while limiting exposure of the products to basic reaction conditions, which have been found to cause rapid decomposition. In addition to improved yields, this work has the added advantage of ease of processing, increased safety profile, and scale-up potential.
Article PDF
Similar content being viewed by others
References
Wegner, J.; Ceylan, S.; Kirschning, A. Adv. Synth. Cat. 2012, 354, 17.
Wirth, T. Microreactors in Organic Synthesis and Catalysis; Wiley-VCH: Weinheim, 2008; vol. 14.
Baxendale, I. R.; Brocken, L.; Mallia, C. J. Green Process. Synth. 2013, 2, 211.
Pastre, J. C.; Browne, D. L.; Ley, S. V. Chem. Soc. Rev. 2013, 42, 8849.
Webb, D.; Jamison, T. F. Chem. Sci. 2010, 1, 675.
McQuade, D. T.; Seeberger, P. H. J. Org. Chem. 2013, 78, 6384.
Hartman, R. L.; McMullen, J. P.; Jensen, K. F. Angew. Chem., Int. Ed. 2011, 50, 7502.
Gutmann, B.; Cantillo, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2015, 54, 6688.
Ley, S. V.; Fitzpatrick, D. E.; Ingham, R. J.; Myers, R. M. Angew. Chem., Int. Ed. 2015, 54, 3449.
Porta, R.; Benaglia, M.; Puglisi, A. Org. Pro. Res. Dev. 2016, 20, 2.
Deadman, B. J.; Collins, S. G.; Maguire, A. R. Chem. Eur. J. 2015, 21, 2298.
Müller, S. T. R.; Wirth, T. ChemSusChem 2015, 8, 245.
Malet-Sanz, L.; Susanne, F. J. Med. Chem. 2012, 55, 4062.
Battilocchio, C.; Feist, F.; Hafner, A.; Simon, M.; Tran, D. N.; Allwood, D. M.; Blakemore, D. C.; Ley, S. V. Nat. Chem. 2016, 8, 360.
Li, J.; Ballmer, S. G.; Gillis, E. P.; Fujii, S.; Schmidt, M. J.; Palazzolo, A. M. E.; Lehmann, J. W.; Morehouse, G. F.; Burke, M. D. Science 2015, 347, 1221.
Adamo, A.; Beingessner, R. L.; Behnam, M.; Chen, J.; Jamison, T. F.; Jensen, K. F.; Monbaliu, J.-C. M.; Myerson, A. S.; Revalor, E. M.; Snead, D. R.; Stelzer, T.; Weeranoppanant, N.; Wong, S. Y.; Zhang, P. Science 2016, 352, 61.
Tsubogo, T.; Oyamada, H.; Kobayashi, S. Nature 2015, 520, 329.
Deadman, B. J.; O’Mahony, R. M.; Lynch, D.; Crowley, D. C.; Collins, S. G.; Maguire, A. R. Org. Biomol. Chem. 2016, 14, 3423.
Tarrant, E.; O’Brien, C. V.; Collins, S. G. RSC Adv. 2016, 6, 31202.
Ford, A.; Miel, H.; Ring, A.; Slattery, C. N.; Maguire, A. R.; McKervey, M. A. Chem. Rev. 2015, 115, 9981.
Müller, S. T. R.; Murat, A.; Hellier, P.; Wirth, T. Org. Pro. Res. Dev. 2016, 20, 495.
Müller, S. T. R.; Murat, A.; Maillos, D.; Lesimple, P.; Hellier, P.; Wirth, T. Chem. Eur. J. 2015, 21, 7016.
Regitz, M. Angew. Chem., Int. Ed. 1967, 6, 733.
Hodson, D.; Holt, G. J. Chem. Soc. C: Org. 1968, 1602.
Maguire, A. R.; Collins, S. G.; Ford, A. Arkivoc 2003, 7, 96.
Maguire, A. R.; Kelleher, P. G.; Ferguson, G.; Gallagher, J. F. Tetrahedron Lett. 1998, 39, 2819.
Collins, S. G.; O’Sullivan, O. C. M.; Kelleher, P. G.; Maguire, A. R. Org. Biomol. Chem. 2013, 11, 1706.
O’Sullivan, O. C. M.; Collins, S. G.; Maguire, A. R.; Böhm, M.; Sander, W. Eur. J. Org. Chem. 2006, 2006, 2918.
O’Sullivan, O.; Collins, S.; Maguire, A. Synlett 2008, 2008, 659.
Sander, W.; Strehl, A.; Maguire, A. R.; Collins, S. G.; Kelleher, P. G. Eur. J. Org. Chem. 2000, 2000, 3329.
O’Sullivan, O. C. M.; Collins, S. G.; Maguire, A. R.; Buche, G. Eur. J. Org. Chem. 2014, 2014, 2297.
Zwanenburg, B. J. Sulfur Chem. 2013, 34, 142.
Zwanenburg, B. Phosphorus, Sulfur Silicon Relat. Elem. 1989, 43, 1.
Zwanenburg, B. Recl. Trav. Chim. Pays-Bas 1982, 101, 1.
Zwanenburg, B.; Damen, T. J. G.; Philipse, H. J. F.; De Laet, R. C.; Lucassen, A. C. B. Phosphorus, Sulfur Silicon Relat. Elem. 1999, 153, 119.
McCaw, P. G.; Buckley, N. M.; Collins, S. G.; Maguire, A. R. Eur. J. Org. Chem. 2016, 2016, 1630.
Maguire, A. R.; Kelleher, P. G.; Lawrence, S. E. Tetrahedron Lett. 1998, 39, 3849.
Hazen, G. G.; Weinstock, L. M.; Connell, R.; Bollinger, F. W. Synth. Commun. 1981, 11, 947.
Mándity, I. M.; Ötvös, S. B.; Fülöp, F. ChemistryOpen 2015, 4, 212.
Ley, S. V.; Baxendale, I. R.; Bream, R. N.; Jackson, P. S.; Leach, A. G.; Longbottom, D. A.; Nesi, M.; Scott, J. S.; Storer, R. I.; Taylor, S. J. J. Chem. Soc., Perk. Trans. 1 2000, 3815.
Kupracz, L.; Hartwig, J.; Wegner, J.; Ceylan, S.; Kirschning, A.; Beilstein, J. Org. Chem. 2011, 7, 1441.
Bihani, M.; Bora, P. P.; Bez, G.; Askari, H. Comp. Rend. Chim. 2013, 16, 419.
Bonfils, F.; Cazaux, I.; Hodge, P.; Caze, C. Org. Biomol. Chem. 2006, 4, 493.
Tamborini, L.; Romanom D.; Pinto, A.; Bertolani, A.; Molinari, F.; Conti, P. J. Mol. Catal. B: Enzym. 2012, 84, 78.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
McCaw, P.G., Deadman, B.J., Maguire, A.R. et al. Delivering Enhanced Efficiency in the Synthesis of α-Diazosulfoxides by Exploiting the Process Control Enabled in Flow. J Flow Chem 6, 226–233 (2016). https://doi.org/10.1556/1846.2016.00013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/1846.2016.00013