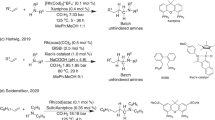
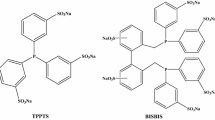
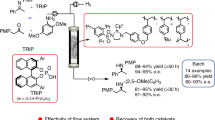
Abstract
This review focuses on the development of continuous rhodium-catalyzed hydroformylations in the past 15 years. Recent progresses are discussed and compared in detail, based on different types of catalyst handling including single-phase homogeneous system, multiphase system, immobilized catalysts on solid supports, supported ionic liquid phase catalyst, and scCO2-ionic liquid biphasic system.
Article PDF
Similar content being viewed by others
References
For a recent review, see: Franke, R.; Selent, D.; Börner, A. Chem. Rev. 2012, 112, 5675–5732.
Farnetti, E.; Di Monte, R.; Kaspar, J. Homogeneous and Heterogeneous Catalysis in Inorganic and Bioinorganic Chemistry; Bertin, I. Eds., EOLSS, Paris, France, 2009; vol. 2
Han, D.; Li, X.; Zhang, H.; Liu, Z.; Li, J.; Li, C. J. Catal. 2006, 243, 318–328
Kainulainen, T. A.; Niemelä, M. K.; Krause, A. O. I. J. Mol. Catal. A 1997, 122, 39–49
Mukhopadhyay, K.; Chaudhari, R. V. J. Catal. 2003, 213, 73–77
Nozaki, K.; Itoi, Y.; Shibahara, F.; Shirakawa, E.; Ohta, T.; Takaya, H.; Hiyama, T. J. Am. Chem. Soc. 1998, 120, 4051–052.
Matsumoto, M.; Tamura, M. J. Mol. Catal. 1983, 19, 365–376.
Fraile, J. M.; Garcia, J. I.; Mayoral, J. A. Chem. Rev. 2009, 109, 360–417.
Lu, J.; Toy, P. H. Chem. Rev. 2009, 109, 815–838.
Cornils, B. Org. Proc. Res. Dev. 1998, 2, 121–127.
Hershman, A.; Robinson, K. K.; Craddock, J. H.; Roth, J. F. Ind. Eng. Chem. Prod. Res. Dev. 1969, 8, 372–375.
Claridge, J. B.; Douthwaite, R. E.; Green, M. L. H.; Lago, R. M.; Tsang, S. C.; York, A. P. E. J. Mol. Catal. 1994, 89, 113–120.
Abatjoglou, A. G.; Billig, E.; Bryant, D. R. Organometallics 1984, 3, 923–926.
Kohlpaintner, C. W.; Fischer, R. W.; Cornils, B. Appl. Catal. A Gen. 2001, 221, 219–225.
Herrmann, W. A.; Cornils, B. Angew. Chem., Int. Ed. 1997, 36, 1048–1067.
McQuade, D. T.; Seeberger, P. H. J. Org. Chem. 2013, 78, 6384–6389
Wiles, C.; Watts, P. Green Chem. 2012, 14, 38–54.
Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wan Q. ChemSusChem, 2013, 6, 746–789.
O’Brien, M.; Taylor, N.; Polyzos, A.; Baxendale, I. R.; Ley, S. V. Chem. Sci. 2011, 2, 1250–1257.
Kasinathan, S.; Bourne, S. L.; Tolstoy, P.; Koos, P.; O’Brien, M.; Bates, R. W.; Baxendale, I. R.; Ley, S. V. Synlett 2011, 18, 2648–2651.
Bourne, S. L.; O’Brien, M.; Kasinathan, S.; Koos, P.; Tolstoy, P.; Hu, D. X.; Bates, R. W.; Martin, B.; Schenkel, B.; Ley, S. V. ChemCatChem 2013, 5, 159–172.
Güven, S.; Hamers, B.; Franke, R.; Priske, M.; Becker, M.; Vogt, D. Catal. Sci. Technol. 2014, 4, 524–530.
Horvath, I. T.; Kiss, G.; Cook, R. A.; Bond, J. E.; Stevens, P. A.; Rabai, J.; Mozeleski, E. J. J. Am. Chem. Soc. 1998, 120, 3133–3143.
Horvath, I. T.; Rabai, J. Science 1994, 266, 72–75.
Perperi, E.; Huang, Y.; Angeli, P.; Manos, G.; Mathison, C. R.; Cole-Hamilton, D. J.; Adams, D. J.; Hope, E. G. Dalton Trans. 2004, 2062–2064.
Rost, A.; Müller, M.; Hamerla, T.; Kasaka, Y.; Wozny, G.; Schomäker, R. Chem. Eng. Process. 2013, 67, 130–135.
Müller, M.; Kasaka, Y.; Müller, D.; Schomäcker, R.; Wozny, G. Ind. Eng. Chem. Res. 2013, 52, 7259–7264.
Beller, M.; Cornils, B.; Frohning, C. D.; Kohlpainter, C. W. J. Mol. Catal. A: Chem. 1995, 104, 17–85.
Heinrich, B.; Hjortkjaer, J.; Nikitidis, A.; Andersson, C. J. Mol. Catal. 1993, 81, 333–347.
Andersson, C.; Nikitidis, A.; Hjortkjaer, J.; Heinrich, B. Appl. Catal. A Gen. 1993, 96, 345–354.
Meehan, N. J.; Sandee, A. J.; Reek, J. N. H.; Kamer, P. C. J.; Van Leeuwen, P. W. N. M.; Poliakoff, M. Chem. Commun. 2000, 1497–1498.
For reviews on continuous flow processes using scCO2 and supported catalysts, see: (a) Stouten, S. C.; Noël, T.; Wang, Q.; Hessel, V. Chem. Eng. Process. 2014, 83, 26–32
Burguete, M. I.; Garcia-Verdugo, E.; Luis, S. V. Beilstein J. Org. Chem. 2011, 7, 1347–1359.
Bronger, R. P. J.; Bermon, J. P.; Reek, J. N. H.; Kamer, P. C. J.; Van Leeuwen, P. W. N. M.; Carter, D. N.; Licence, P.; Poliakoff, M. J. Mol. Catal. A: Chem. 2004, 224, 145–152.
Sakai, N.; Mano, S.; Nozaki, K.; Takaya, H. J. Am. Chem. Soc. 1993, 115, 7033–7034.
Shibahara, F.; Nozaki, K.; Hiyama, T. J. Am. Chem. Soc. 2003, 125, 8555–8560.
Schmidt-Winkel, P.; Lukens, W. W.; Yang, P.-D.; Margolese, D. I.; Lettow, J. S.; Ying, J. Y.; Stucky, G. D. Chem. Mater. 2000, 12, 686–696.
Yan, L.; Ding, Y.-J.; Zhu, H.; Yin, H.-M.; Jiao, G.-P.; Zhao, D.-Y.; Lin, L.-W. Chin. J. Catal. 2006, 27, 1–3.
Janssen, M.; Müller, C.; Vogt, D. Dalton Trans. 2010, 39, 8403–8411.
Janssen, M.; Wilting, J.; Müller, C.; Vogt, D. Angew. Chem., Int. Ed. 2010, 49, 7738–7741.
Fang, J.; Jana, R.; Tunge, J. A.; Subramaniam, B. Appl. Catal. A Gen. 2011, 393, 294–301.
Schönweiz, A.; Debuschewitz, J.; Walter, S.; Wölfel, R.; Hahn, H.; Dyballa, K. M.; Franke, R.; Haumann, M.; Wasserscheid, P. ChemCatChem 2013, 5, 2955–2963.
Kamer, P. C. J.; Reek, J. N. H.; van Leeuwen P. W. N. M. in Rhodium Catalyzed Hydroformylation; van Leeuwen, P. W. N. M., Claver, C. Eds.; Kluwer Academic Publisher.; Dordrecht, 2000, 35–62.
Cornils, B.; Wiebus, E. Chemtech 1995, 25, 33–38.
Arhancet, J. P.; Davis, M. E.; Merola, J. S.; Hanson, B.E. Nature 1989, 339, 454–455.
Wasserscheid, P.; van Hal, R.; Bosmann, A. Green Chem. 2002, 4, 400–404.
Wasserscheid, P.; Waffenschmidt, H.; Machnitzki, P.; Kottsieper; K.W.; Stelzer, O. Chem. Commun. 2001, 451–452.
Mehnert, C. P.; Cook, R. A.; Dispenziere, N. C.; Afeworki, M. J. Am. Chem. Soc. 2002, 124, 12932–12933.
Riisager, A.; Eriksen, K. M.; Wasserscheid, P.; Fehrmann, R. Catal. Lett. 2003, 90, 149–153
Hjortkjaer, J.; Chen, Y.-Y.; Heinrich, B. Appl. Catal. 1991, 67, 269–278.
Riisager, A.; Wasserscheid, P.; van Hal, R.; Fehrmann, R. J. Catal. 2003, 219, 452–455.
Dupont, J.; Silva, S. M.; de Souza, R. F. Catal. Lett. 2001, 77, 131–133.
Riisager, A.; Fehrmann, R.; Flicker, S.; van Hal, R.; Haumann, M.; Wasserscheid, P. Angew. Chem., Int. Ed. 2005, 44, 815–819.
Haumann, M.; Dentler, K.; Joni, J.; Riisager, A.; Wasserscheid, P. Adv. Synth. Catal. 2007, 349, 425–431.
Haumann, M.; Jakuttis, M.; Franke, R.; Schönweiz, A.; Wasserscheid, P. ChemCatChem 2011, 3, 1822–1827.
Behr, A.; Obst, D.; Schulte, C.; Schosser, T. J. Mol. Catal. A: Chem. 2003, 206, 179–184.
Blanchard, L. A.; Hancu, D.; Beckman, E. J.; Brennecke, J. F. Nature 1999, 399, 28–29.
Blanchard, L. A.; Brennecke, J. F. Ind. Eng. Chem. Res. 2001, 40, 287–292.
Kainz, S.; Leitner, W. Catal. Lett. 1998, 55, 223–225.
Koch, D.; Leitner, W. J. Am. Chem. Soc. 1998, 120, 13398–13404.
Palo, D. R.; Erkey, C. Ind. Eng. Chem. Res. 1998, 37, 4203–206.
Palo, D. R.; Erkey, C. Organometallics 2000, 19, 81–86.
Sellin, M. F.; Webb, P. B.; Cole-Hamilton, D. J. Chem. Commun. 2001, 781–782.
Webb, P. B.; Sellin, M. F.; Kunene, T. E.; Williamson, S.; Slawin, A. M. Z.; Cole-Hamilton, D. J. J. Am. Chem. Soc. 2003, 125, 15577–15588.
Kunene, T. E.; Webb, P. B.; Cole-Hamilton, D. J. Green Chem. 2011, 13, 1476–1481.
Frisch, A. C.; Webb, P. B.; Zhao, G.-Y.; Muldoon, M. J.; Pogorzelec, P. J.; Cole-Hamilton, D. J. Dalton Trans. 2007, 5531–5538.
Hintermair, U.; Gong, Z.-X.; Serbanovic, A.; Muldoon, M. J.; Santini, C. C.; Cole-Hamilton, D. J. Dalton Trans. 2010, 39, 8501–8510.
Hintermair, U.; Zhao, G.-Y.; Santini, C. C.; Muldoon, M. J.; Cole-Hamilton D. J. Chem. Commun. 2007, 1462–1464.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X. Recent Advances in Continuous Rhodium-Catalyzed Hydroformylation. J Flow Chem 5, 125–132 (2015). https://doi.org/10.1556/1846.2015.00003
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/1846.2015.00003