Abstract
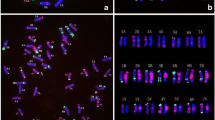
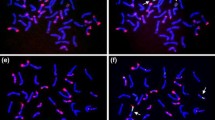
The 4H(4D) wheat/barley substitution line was crossed with the ‘Chinese Spring’ ph1b mutant genotype in order to induce wheat-barley homoeologous recombinations. F3 and F4 seeds of the 4H(4D) × ‘Chinese Spring’ ph1b mutant cross were analysed using genomic in situ hybridization, and a Robertsonian translocation was detected in monosomic form. Disomic centric fusions were selected among the self-fertilized progenies. The presence of the long arm of 4H was confirmed with SSR markers. The long arm of the 5D wheat chromosome in the Robertsonian translocation was identified using fluorescent in situ hybridization with the help of three DNA probes: pSc119.2, Afa family and pTa71. The wheat/barley centric fusion was identified as a 4HL.5DL translocation. This line exhibited supernumerary spikelet character, but the number of seeds/plant did not increase. The 4HL.5DL centric fusion line is suitable genetic material to study the expression of genes located on 4HL in a wheat genetic background.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Bedbrook, J., Jones, J., O’Dell, M., Thompson, R.D., Flavell, R.B. 1980. A molecular description of telomeric heterochromatin in Secale species. Cell 19:545–560.
Chauhan, T., Singh, B.M. 1994. Karnal Bunt resistance in wheat-barley addition lines. Plant Breed. 112:252–255.
Colas, I., Shaw, P., Prieto, P., Wanous, M., Spielmeyer, W., Mago, R., Moore, G. 2008. Effective chromosome pairing requires chromatin remodelling at the onset of meiosis. Proc. Natl. Acad. Sci. 105:6075–6080.
Cseh, A., Kruppa, K., Molnár, I., Rakszegi, M., Dolezel, J., Molnár-Láng, M. 2011. Characterization of a new 4BS.7HL wheat-barley translocation line using GISH, FISH, and SSR markers and its effect on the b-glucan content of wheat. Genome 54:1–10.
Friebe, B., Jiang, J., Raupp, W.J., McIntosh, R.A., Gill, B.S. 1996. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 91:59–87.
Gale, M.D., Miller, T.E. 1987. The introduction of alien genetic variation in wheat. In: Lupton, F.G.H. (ed.), Wheat Breeding: Its Scientific Basis. Chapman and Hall, London, UK, pp. 173–210.
Gerlach, W.L., Bedbrook, J.L. 1979. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 7:1869–1885.
Griffiths, S., Sharp, R., Foote, T.N., Bertin, I., Wanous, M., Reader, S., Colas, I., Moore, G. 2006. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439:749–752.
Handley, L.L., Nevo, E., Raven, J.A., Martýnez-Carrasco, R., Scrimgeour, C.M., Pakniyat, H., Forster, B.P. 1994. Chromosome 4 controls potential water use efficiency (d13C) in barley. J. Exp. Bot. 45:1661–1663.
Hart, G.E., Islam, A.K.M.R., Shepherd, K.W. 1980. Use of isozymes as chromosome markers in the isolation and characterization of wheat-barley chromosome addition lines. Genet. Res. 36:311–326.
Hoffmann, B., Aranyi, N.R., Molnár-Láng, M. 2010. Characterization of wheat-barley introgression lines for drought tolerance. Acta Agron. Hung. 58:211–218.
Islam, A.K.M.R., Shepherd, K.W. 1992. Production of wheat-barley recombinant chromosomes through induced homoeologous pairing 1. Isolation of recombinants involving barley arms 3HL and 6HL. Theor. Appl. Genet. 83:489–494.
Jauhar, P.P., Chibbar, R.N. 1999. Chromosome-mediated and direct gene transfers in wheat. Genome 42:570–583.
Karsai, I., Mészáros, K., Szûcs, P., Hayes, P.M., Láng, L., Bedõ, Z. 2006. The influence of photoperiod on the Vrn-H2 locus (4H) which is a major determinant of plant development and reproductive fitness traits in a facultative × winter barley (Hordeum vulgare L.) mapping population. Plant Breed. 125:468–472.
Koebner, R.M.D., Shepherd, K.W. 1985. Induction of recombination between rye chromosome 1RL and wheat chromosomes. Theor. Appl. Genet. 71:208–215.
Kreis, M., Williamson, M.S., Shewry, P.R., Sharp P., Gale, M. 1988. Identification of a second locus encoding b-amylase on chromosome 2 of barley. Genet. Res. 51:13–16.
Molnár, I., Linc, G., Dulai, S., Nagy, E.D., Molnár-Láng, M. 2007. Ability of chromosome 4H to compensate for 4D in response to drought stress in a newly developed and identified wheat-barley 4H(4D) disomic substitution line. Plant Breed. 126:369–374.
Molnár-Láng, M., Sutka, J. 1994. The effect of temperature on seed set and embryo development in reciprocal crosses of wheat and barley. Euphytica 78:53–58.
Molnár-Láng, M., Linc, G., Friebe, B.R., Sutka, J. 2000. Detection of wheat-barley translocations by genomic in situ hybridization in derivatives of hybrids multiplied in vitro. Euphytica 112:117–123.
Molnár-Láng, M., Kruppa, K., Cseh, A., Bucsi, J., Linc, G. 2012. Identification and phenotypic description of new wheat/six-rowed winter barley disomic additions. Genome 55:302–311.
Murai, K., Koba, T., Shimada, T. 1997. Effects of barley chromosome on heading characters in wheat-barley chromosome addition lines. Euphytica 96:281–287.
Nagaki, K., Tsujimoto, H., Isono, K., Sasakuma, T. 1995. Molecular characterization of a tandem repeat, Afa family, and its distribution among Triticeae. Genome 38:479–486.
Oberthur, L., Dyer, W., Ullrich, S.E., Blake, T.K. 1995. Genetic analysis of seed dormancy in barley (Hordeum vulgare L.). J Quant Trait Loci. Available on the internet: https://doi.org/www.ncgr.org/research/jag/papers95/paper595/indexp595.html
Riley, R., Chapman, V. 1958. Genetic control of cytologically diploid behaviour of hexaploid wheat. Nature 182:713–715.
Riley, R., Chapman, V., Johnson, R. 1968. Introduction of yellowrust resistance of Aegilops comosa into wheat by genetically induced homoeologous recombination. Nature 217:383–384.
Sears, E.R. 1977. An induced mutant with homoeologous pairing in common wheat. Can. J. Genet. Cytol. 19:585–593.
Sears, E.R. 1981. Transfer of alien genetic material to wheat. In: Evans, L.T., Peacock, W.J. (eds), Wheat Science — Today and Tomorrow. Cambridge University Press, Cambridge, UK, pp. 75–89.
Sepsi, A., Németh, K., Molnár, I., Szakács, É., Molnár-Láng, M. 2006. Induction of chromosome rearrangements in a 4H(4D) wheat-barley substitution using a wheat line containing a Ph suppressor gene. Cereal Res. Commun. 34:1215–1222.
Sharma, H., Ohm, H., Goulart, L., Lister, R., Appels, R., Benlhabib, O. 1995. Introgression and characterization of barley yellow dwarf virus resistance from Thinopyrum intermedium into wheat. Genome 38:406–413.
Sherman, J.D., Smith, L.Y., Blake, T.K., Talbert, L.E. 2001. Identification of barley genome segments introgressed into wheat using PCR markers. Genome 44:38–44.
Taketa, S., Awayama, T., lchii, M., Sunakawa, M., Kawahara, T., Mural, K. 2005. Molecular cytogenetic identification of nullisomy 5B induced homoeologous recombination between wheat chromosome 5D and barley chromosome 5H. Genome 48:115–124.
Tang, Y., Sorrels, M.E., Kochian, L.V., Garvin, D.F. 1999. Identification of RFLP markers linked to the barley aluminium tolerance gene Alp. Crop. Sci. 40:778–782.
Zhu, B., Choi, D.-W., Fenton, R., Close, T.J. 2000. Expression of the barley dehydrin multigene family and the development of freezing tolerance. Mol. Gen. Genet. 264:145–153.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Aniol
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kruppa, K., Türkösi, E., Szakács, É. et al. Development and Identification of a 4HL.5DL Wheat/Barley Centric Fusion Using Gish, Fish and SSR Markers. CEREAL RESEARCH COMMUNICATIONS 41, 221–229 (2013). https://doi.org/10.1556/CRC.2012.0038
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/CRC.2012.0038