Abstract
Cerebrospinal fluid (CSF)-contacting neurons are sensory-type cells sending ciliated dendritic process into the CSF. Some of the prosencephalic CSF-contacting neurons of higher vertebrates were postulated to be chemoreceptors detecting the chemical composition of the CSF, other cells may percieve light as “deep encephalic photoreceptors”. In our earlier works, CSF-contacting neurons of the mechanoreceptor-type were described around the central canal of the hagfish spinal cord. It was supposed that perceiving the flow of the CSF they are involved in vasoregulatory mechanisms of the nervous tissue. In the present work, we examined the brain ventricular system of the Atlantic hagfish with special reference to the presence and fine structure of CSF-contacting neurons.
Myxinoids have an ontogenetically reduced brain ventricular system. In the adult hagfish (Myxine glutinosa) the lumen of the lateral ventricle is closed, the third ventricle has a preoptic-, infundibular and subhabenular part that are not connected to each other. The choroid plexus is absent. The infundibular part of the third ventricle has a medial hypophyseal recess and, more caudally, a paired lateral recess. We found CSF-contacting neurons in the lower part of the third ventricle, in the preoptic and infundibular recess as well as in the lateral infundibular recesses.
No CSF-contacting neurons were found in the cerebral aqueduct connecting the subhabenular recess to the fourth ventricle. There is a pineal recess and a well-developed subcommissural organ at the rostral end of the aqueduct. Extending from the caudal part of the fourth ventricle in the medulla to the caudal end of the spinal cord, the central canal has a dorsal and ventral part. Dendrites of CSF-contacting neurons are protruding into the ventral lumen. Corroborating the supposed choroid plexus-like function of the wall of the dorsal central canal, segmental vessels reach a thin area on both sides of the ependymal lining.
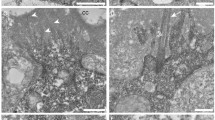
The perikarya of the CSF-contacting neurons found in the brain ventricles are mainly bipolar and contain granular vesicles of various size. The bulb-like terminal of their ventricular dendrites bears several stereocilia and contains basal bodies as well as mitochondria. Basal bodies emit cilia of the 9 +0-type. Cilia may arise from the basal body and accessory basal body as well. The axons run ependymofugally and enter - partially cross - the periventricular synaptic zones. No neurohemal terminals similar to those formed by spinal CSF-contacting neurons of higher vertebrates have been found in the hagfish. We suppose that CSF-contacting neurons transform CSF-mediated non-synaptic information taken up by their ventricular dendrites to synaptic one. A light-sensitive role for some (preoptic?) groups of CSF-contacting neurons cannot be excluded.
Article PDF
Similar content being viewed by others
References
Adam, H. (1956) Der III. Ventrikel und die mikroskopische Struktur seiner Wände bei Lampetra (Petromyzon) fluviatilis L. und Myxine glutinosa nebst einigen Bemerkungen über das Infundibularorgan von Branchiostoma (Amphioxus) lanceolatus Pall. Progr. Neurobiol. (Amst.) 1, 146–158.
Adam, H. (1957) Beitrag zur Kenntnis der Hirnventrikel und des Ependyms bei den Cyclostomen. Anat. Anz. 103, 173–188.
Bone, Q. (1936) The central nervous system. In: Brodal, A., Fänge R. (eds) The Biology of the Myxine. Skandinavian University Books, Oslo, pp. 50–91.
Crosby, E., Schnitzlein, H. N. (1974) The comparative anatomy of the telencephalon of the hagfish, Myxine glutinosa. J. Hirnforsch. 15, 211–236.
Donald, J. A., Toop, T., Evans, D. H. (1999) Natriuretic peptide bindind sites in the brain of the Atlantic hagfish, Myxine glutinosa. J. Exp. Zool. 284, 407–413.
Edinger, L. (1906) Über das Gehirn von Myxine glutinosa. Abh. Königl. Preus. Akad. 190, 1–36.
Foster, R. G., Grace, M. S., Provencio, I., Degrip, W. J., Garcia-Fernandez, J. M. (1994) Identification of vertebrate deep brain photoreceptors. Neurosci. Behav. Rev. 18, 541–546.
Holm, F. (1901) The finer anatomy of the nervous system of Myxine glutinosa. Morphol. Jahrb. 29, 365–401.
Holmgren, N. (1919) Zur Anatomie des Gehirnes von Myxine. Kgl. Svenska Vet.-Akad. Handl. 60, 1–96.
Holmgren, N. (1946) On two embryos of Myxine glutinosa. Acta Zool. (Stockh.) 27, 1–90.
Jansen, J. (1930) The brain of Myxine glutinosa. J. Comp. Neurol. 49, 359–507.
Jirikowski, G., Erhardt, G., Grimmelikhuizen, C. J. P., Triepel, J., Patzner, R. A. (1984) FMRFamide-like immunoreactivity in the brain and pituitary of the hagfish, Eptatretus burgeri (Cyclostomata). Cell Tissue Res. 237, 362–366.
Kadota, T. (1991) Distribution of 5HT (serotonnin) immunoreactivity in the central nervous system of the inshore hagfish, Eptatretus burgeri. Cell Tiss. Res. 266, 107–116.
Kadota, T., Goris, R. C., Kusunoki, T. (1993) Dopamine neurons in the hagfish brain. Anat. Record Suppl. 1, 69.
Olsson, R. (1959) The neurosecretory hypothalamus system and the adenohypophysis of Myxine. Z. Zellforsch. 51, 97–107.
Ooka-Souda, S., Kadota, T., Kabasawa, H. (1993) The preoptic nucleus: the probable location of the circadian pacemaker of the hagfish, Eptatretus burgeri. Neurosci. Lett. 164, 33–36.
Ooka-Souda, S., Kadota, T., Kabasawa, H., Takeuchi, H. (1995) A possible retinal information route to the circadian pacemaker through pretectal areas in the hagfish, Eptatretus burgeri. Neurosci. Lett. 192, 201–204.
Retzius, G. (1893) Das Gehirn und das Auge von Myxine. Biol. Untersuch. N. F. 5, 55–68.
Tsuneki, K. (1986) A survey of occurrence of about seventeen circumventricular organs in brains of various vertebrates with special reference to lower groups. J. Hirnforsch. 27, 441–470.
Ueck, M., Kobayashi, H. (1979) Neue Ergebnisse zu Fragen der vergleichenden Epiphysen-forschung. Verh. Anat. Ges. 73, 961–963.
Vígh, B., Vígh-Teichmann, I. (1971) Structure of the medullo-spinal liquor contacting neuronal system. Acta Biol. Hung. 22, 227–243.
Vígh, B., Vígh-Teichmann, I. (1982) The cerebrospinal fluid-contacting neurosecretory cell: A pro-toneuron. In: Farner, D. S., Lederis, K. (eds) Molecules, Cells Systems. Plenum Press, New York, pp. 458–460.
Vígh, B., Vígh-Teichmann, I. (1991) The double central canal of Myxine glutinosa: presence of CSF-contacting neurons and glial labyrinth. Anat. Anz. (Suppl.) 172, 332.
Vígh, B., Vígh-Teichmann, I. (1992) Cytochemistry of CSF-contacting neurons and pinealocytes. Progr. Brain Res. 91, 299–306.
Vígh, B., Vígh-Teichman, I. (1998) Actual problems of the cerebrospinal fluid contacting neurons. Microsc. Res. Techn. 41, 57–83.
Vígh, B., Vígh-Teichmann, I., Aros, B. (1969) Das Paraventricularorgan und das Liquorkontakt-Neuronensystem. Anat. Anz. 125, 683–688.
Vígh, B., Vígh-Teichmann, I., Koritsánszky, S., Aros, B. (1970) Ultrastruktur der Liquorkontakt-neurone des Rückenmarkes von Reptilien. Z. Zellforsch. 109, 180–194.
Vígh, B., Vígh-Teichmann, I., Aros, B. (1971) Ultrastruktur der spinalen Liquorkontaktneurone beim Krallenfrosch (Xenopus laevis). Z. Zellforsch. 112, 201–211.
Vígh, B., Manzano, M. J., Zádori, A., Frank, C. L., Lukáts, Á., Röhlich, P., Szél, Á., Dávid, C. (2002) Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histol. Histopathol (in press).
Vígh-Teichmann, I., Vígh, B. (1974) The infundibular cerebrospinal fluid-contacting neurons. Ergebn. Anat. Entwickl. Gesch. 50, 1–91.
Vígh-Teichmann, I., Vígh, B. (1983) The system of cerebrospinal fluid contacting neurons. Arch. Histol. Japon. 46, 427–468.
Vígh-Teichmann, I., Vígh, B. (1989) The cerebrospinal fluid-contacting neuron: a peculiar cell type of the central nervous system. Immunocytochemical aspects. Arch. Histol.Cytol. 52, 195–207.
Vígh-Teichmann, I., Vígh, B., Olsson R., Van Veen, Th. (1984) Opsin immunoreactive outer segments of photoreceptors in the eye and in the lumen of the optic nerve of the hagfish, Myxine glutinosa. Cell Tissue Res. 238, 515–522.
Wächtler, K. (1975) The distribution of acetylcholinesterase in the cyclostome brain. II. Myxine glutinosa. Cell Tiss. Res. 159, 109–120.
Wernadakis, A. J., Bemis, W. E., Bittman, E. L. (1998) Localization and partial characterization of melatonin receptors in amphyoxus, hagfish, lamprey, and skate. Gen. Comp. Endocrinol. 110, 67–78.
Wicht, U., Northcutt, R. G. (1992) FMRFamide-like immunoreactivity in the brain of the Pacific hag-fish, Eptatretus stouti. Cell Tissue Res. 270, 443–449.
Wicht, U., Northcutt, R. G. (1994) An immunohistochemical study of the telencephalon and dien-cephalon in a myxinoid jawless fish, the Pacific hagfish, Eptatretus stouti. Brain Behav. Evol. 43, 140–161.
Worthington, J. (1905) Contribution to our knowledge of the myxinoids. Am. Naturalist 39, 625–668.
Acknowledgement
This work was supported by grants from the Hungarian Scientific Research Fund (OTKA), Nos T 032860 and T 29048.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dávid, C., Frank, C.L., Lukáts, Á. et al. Cerebrospinal Fluid Contacting Neurons in the Reduced Brain Ventricular System of the Atlantic Hagfish, Myxine glutinosa. BIOLOGIA FUTURA 54, 35–44 (2003). https://doi.org/10.1556/ABiol.54.2003.1.4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/ABiol.54.2003.1.4