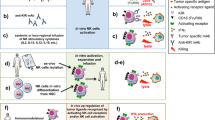
Abstract
Natural killer cell receptor (NKR)-expressing cells have cytolytic activity against leukemic cells, and solid tumor cells escape from T-cell recognition because of the low expression levels of class I HLA molecules in both allogeneic and autologous settings. This characteristic feature of NK cell recognition of target cells in contrast with that of T-cells provides a strategy to overcome tolerance in the tumor-bearing host. Furthermore, inhibitory NKR-expressing cells may have cytolytic activity and immunoregulatory functions. Several methods can be used to expand NKR-expressing cells for adoptive immunotherapy for leukemia and other malignant diseases.We review recent developments in the biology and clinical application of NKR-expressing cells, such as NK cells, lymphokine-activated killer cells, cytokine-induced killer cells, NKT cells, and other NKR-expressing cells.
Similar content being viewed by others
References
Farag SS, VanDeusen JB, Fehniger TA, Caligiuri MA. Biology and clinical impact of human natural killer cells. Int J Hematol. 2003;78:7–17.
Imamura M, Tanaka J. Immunoregulatory cells for transplantation tolerance and graft-versus-leukemia effect. Int J Hematol. 2003;78:188–194.
Voutsadakis IA. NK cells in allogeneic bone marrow transplantation. Cancer Immunol Immunother. 2003;52:525–534.
Shibuya A. Development and functions of natural killer cells. Int J Hematol. 2003;78:1–6.
Young NT. Immunobiology of natural killer lymphocytes in transplantation. Transplantation. 2004;78:1–6.
Ljunggren HG, Karre K. In search of the "missing self":MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244.
Moretta A, Biassoni R, Bottino C, et al. Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol Rev. 1997;155:105–117.
Bakker ABH, Phillips JH, Figdor CG, Lanier LL. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, γ δ T cells, and antigen-specific CTL. J Immunol. 1998;160:5239–5245.
Phillips JH, Gumperz JE, Parham P, Lanier LL. Superantigendependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995;268:403–405.
Mingari MC, Schiavetti F, Ponte M, et al.. Human CD8+ T lymphocyte subsets that express HLA class I-specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc Natl Acad Sci U S A. 1996;93:12433–12438.
Huard B, Karlsson L. KIR expression on self-reactive CD8+ T cells is controlled by T-cell receptor engagement. Nature. 2000;403:325–328.
Mingari MC, Ponte M, Bertone S, et al. HLA class I-specific inhibitory receptors in human T lymphocytes:interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigenactivated CD8+ T cells. Proc Natl Acad Sci U S A. 1998;95:1172–1177.
Mingari MC, Moretta A, Moretta L. Regulation of KIR expression in human T cells:a safe mechanism that may impair protective T-cell responses. Immunol Today. 1998;19:153–157.
Lowdell MW, Lamb L, Hoyle C, Velardi A, Prentice HG. Non- MHC-restricted cytotoxic cells:their roles in the control and treatment of leukaemias. Br J Haematol. 2001;114:11–24.
Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caliggiuri MA. Nat ural killer cell receptors:new biology and insights into the graftversus- leukemia effect. Blood. 2002;100:1935–1947.
Colonna M, Borsellino G, Falco M, Ferrara GB, Strominger JL. HLA-C is the inhibitory ligand that determines dominant resistance to lysis by NK1- and NK2-specific natural killer cells. Proc Natl Acad Sci U S A. 1993;90:12000–12004.
Biassoni R, Cantoni C, Falco M, et al. The human leukocyte antigen (HLA)-C-specific "activatory" or "inhibitory" natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183:645–650.
Lanier LL. Activating and inhibitory NK cell receptors. Adv Exp Med Biol. 1998;452:13–18.
Ulbrecht M, Honka T, Person S, Johnson JP, Weiss EH. The HLA-E gene encodes two differentially regulated transcripts and a cell surface protein. J Immunol. 1992;149:2945–2953.
Borrego F, Ulbrech M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813–818.
Braud VM, Allan DSJ, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799.
Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790.
Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171.
Wu J, Song Y, Bakker AB, et al. An activating immunoreceptor complexformed by NKG2D and DAP10. Science. 1999;285:730–732.
Bahram S. MIC genes:from genetics to biology. Adv Immunol. 2000;76:1–60.
Sutherland CL, Chalupny NJ, Cosman D. The UL16-binding proteins, a novel family of MHC class I-related ligands for NKG2D, activate natural killer cell functions. Immunol Rev. 2001;181:185–792.
Pietra G, Romagnani C, Mazzarino P, et al. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci U S A. 2003;100:10896- 10901.
Farag SS, George SL, Lee EJ, et al. Postremission therapy with lowdose interleukin 2 with or without intermediate pulse dose interleukin 2 therapy is well tolerated in elderly patients with acute myeloid leukemia:Cancer and Leukemia Group B Study 9420. Clin Cancer Res. 2002;8:2812–2819.
Pende D, Cantoni C, Rivera P, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells:cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086.
Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8α β T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260.
Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729.
Cosman D, Mullberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133.
Garrido F, Cabrera T, Lopez-Nevot MA, Ruiz-Cabello F. HLA class I antigens in human tumors. Adv Cancer Res. 1995;67:155–195.
Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition:molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273.
Brouwer RE, van der Heiden P, Schreuder GM, et al. Loss or downregulation of HLA class I expression at the allelic level in acute leukemia is infrequent but functionally relevant, and can be restored by interferon. Hum Immunol. 2002;63:200–210.
Amiot L, Onno M, Lamy T, et al. Loss of HLA molecules in B lymphomas lymphomas is associated with an aggressive clinical course. Br J Haematol. 1998;100:655–663.
Savoia P, D’Alfonso S, Peruccio D, et al. Loss of surface HLA class I molecules in leukemic myeloblasts is correlated with an increased leukocyte concentration at onset. Haematologica. 1992;77:127–129.
Demanet C, Mulder A, Deneys V, et al. Down-regulation of HLA-A and HLA-Bw6, but not HLA-Bw4, allospecificities in leukemic cells:an escape mechanism from CTL and NK attack? Blood. 2004;103:3122–3130.
Albi N, Ruggeri L, Aversa F, et al. Natural killer (NK)-cell function and antileukemic activity of a large population of CD3+/CD8+ T cells expressing NK receptors for major histocompatibility complex class I after "three-loci" HLA-incompatible bone marrow transplantation. Blood. 1996;87:3993–4000.
Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339.
Cambiaggi A, Verthuy C, Naquet P, et al. Natural killer cell acceptance of H-2 mismatch bone marrow grafts in transgenic mice expressing HLA-Cw3 specific killer cell inhibitory receptor (CD158b). Proc Natl Acad Sci U S A. 1997;94:8088–8092.
Cambiaggi A, Darche S, Guia S, Kourilsky P, Abastado JP, Vivier E. Modulation of T-cell functions in KIR2DL3 (CD158b) transgenic mice. Blood. 1999;94:2396–2402.
Petersdorf EW, Longton GM, Anasetti C, et al. Association of HLA-C disparity with graft failure after marrow transplantation from unrelated donors. Blood. 1997;89:1818–1823.
Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor:Japan Marrow Donor Program. N Engl J Med. 1998;339:1177–1185.
Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100.
Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants:killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827.
Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819.
Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650.
Cook MA, Milligan DW, Fegan CD, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103:1521–1526.
Igarashi T, Wynberg J, Srinivasan R, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170–177.
Tanaka J, Mori A, Ohta S, et al. Expression of HLA-C-specific natural killer cell receptors (CD158a and CD158b) on peripheral blood mononuclear cells after allogeneic bone marrow transplantation. Br J Haematol. 2000;108:778–783.
Tanaka J, Mori A, Ohta S, Kobayashi S, Asaka M, Imamura M. Sequential analysis of HLA-C-specific killer cell inhibitory receptor (CD158b) expressing peripheral blood mononuclear cells during chronic graft-versus-host disease. Bone Marrow Transplant. 2000;26:287–290.
Tanaka J, Tsutsumi Y, Zhang L, et al. Increased expression of HLAclass- I-specific killer cell inhibitory receptors (CD94) on peripheral blood mononuclear cells after allogeneic bone marrow transplantation. Acta Haematol. 2001;105:89–91.
Vitale C, Pitto A, Benvenuto F, et al. Phenotypic and functional analysis of the HLA-class I-specific inhibitory receptors of natural killer cells isolated from peripheral blood of patients undergoing bone marrow transplantation from matched unrelated donors. Hematol J. 2000;1:136–144.
Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003; 101:3730–3740.
Malmberg KJ. Effective immunotherapy against cancer:a question of overcoming immune suppression and immune escape? Cancer Immunol Immunother. 2004;53:879–892.
Mapara MY, Sykes M. Tolerance and cancer:mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004; 22:1136–1151.
Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332.
Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287.
Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma:direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644.
Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–273.
Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854.
Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokineactivated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897.
Law TM, Motzer RJ, Mazumdar M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824–832.
Kimura H, Yamaguchi Y. A phase III randomized study of interleukin- 2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer. 1997;80:42–49.
Meropol NJ, Barresi GM, Fehniger TA, Hitt J, Franklin M, Caligiuri MA. Evaluation of natural killer cell expansion and activation in vivo with daily subcutaneous low-dose interleukin-2 plus periodic intermediate-dose pulsing. Cancer Immunol Immunother. 1998;46:318–326.
Dhanji S, Teh HS. IL-2-activated CD8+CD44high cells express both adaptive and innate immune system receptors and demonstrate specificity for syngeneic tumor cells. J Immunol. 2003;171:3442–3450.
Koh CY, Blazar BR, George T, et al. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood. 2001;97:3132–3127.
Lowdell MW, Craston R, Samuel D, et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol. 2002;117:821–827.
Romagnani C, Pietra G, Falco M, et al. Identification of HLA-Especific alloreactive T lymphocytes:a cell subset that undergoes preferential expansion in mixed lymphocyte culture and displays a broad cytolytic activity against allogeneic cells. Proc Natl Acad Sci U S A. 2002;99:11328–11333.
Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002; 419:734–738.
Salih HR, Antropius H, Gieseke F, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396.
Maccalli C, Pende D, Castelli C, Mingari MC, Robbins PF, Parmiani G. NKG2D engagement of colorectal cancer-specific T cells strengthens TCR-mediated antigen stimulation and elicits TCR independent antitumor activity. Eur J Immunol. 2003;33:2033–2043.
Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072.
Dunne J, Lynch S, O’Farrelly C, et al. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol. 2001;167:3129–3138.
Derre L, Corvaisier M, Pandolfino MC, Diez E, Jotereau F, Gervois N. Expression of CD94/NKG2-A on human T lymphocytes is induced by IL-12:implications for adoptive immunotherapy. J Immunol. 2002; 168:4864–4870.
Sivori S, Cantoni C, Parolini S, et al. IL-21 induces both rapid maturation of human CD34+ cell precursors towards NK cells and acquisition of surface killer Ig-like receptors. Eur J Immunol. 2003; 33:3439–3447.
Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3+CD56+ cytotoxic cells from chronic myeloid leukemia patients:in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92:3318–3327.
Verneris MR, Baker J, Edinger M, Negrin RS. Studies of ex vivo activated and expanded CD8+ NK-T cells in humans and mice. J Clin Immunol. 2002;22:131–136.
Tanaka J, Toubai T, Tsutsumi Y, et al. Cytolytic activity and regulatory functions of inhibitory NK cell receptor-expressing T cells expanded from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Blood. 2004;104:768–774.
Beresford PJ, Xia Z, Greenberg AH, Lieberman J. Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity. 1999;10:585–594.
Tsutsumi Y, Tanaka J, Sugita J, et al. Analysis of T cell repertoire and mixed chimerism in a patient with aplastic anemia after allogeneic bone marrow transplantation. Br J Haematol. 2002;118:136–139.
Wang J, Springer TA. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol Rev. 1998;163:197–215.
Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366.
Tanaka J, Tsutsumi Y, Zhang L, et al. Induction of CD94/NKG2A expression on T cells in mixed lymphocyte culture by CD14+ cells from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Br J Haematol. 2002;117:751–754.
Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295.
Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCRmediated activation of V_14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629.
Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in nonobese diabetic mice. Nat Med. 2001;7:1052–1056.
Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat Med. 2001;7:1057–1062.
Rigby SM, Rouse T, Field EH. Total lymphoid irradiation nonmyeloablative preconditioning enriches for IL-4-producing CD4+-TNK cells and skews differentiation of immunocompetent donor CD4+ cells. Blood. 2003;101:2024–2032.
Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graftversus- host disease induction due to interferon γ production. Blood. 2001;97:2923–2931.
Takahashi T, Haraguchi K, Chiba S, Yasukawa M, Shibata Y, Hirai H. Vα24+ natural killer T-cell responses against T-acute lymphoblastic leukaemia cells:implications for immunotherapy. Br J Haematol. 2003;122:231–239.
Haraguchi K, Takahashi T, Hiruma K, et al. Recovery of Vα24+ NKT cells after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:595–602.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tanaka, J., Asaka, M. & Imamura, M. Potential Role of Natural Killer Cell Receptor-Expressing Cells in Immunotherapy for Leukemia. Int J Hematol 81, 6–12 (2005). https://doi.org/10.1532/IJH97.04152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1532/IJH97.04152