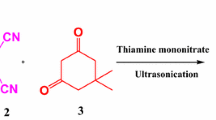
Abstract
Xanthenedione derivatives were synthesised in one-pot reactions between arylaldehyde derivatives and 1,3-cyclohexanedione promoted by niobium pentachloride. This new method is simple, costeffective, high-yielding with a good variety of substrates generality, and can be conducted within reasonable reaction times.
Similar content being viewed by others
References
Ahmad, M., King, T. A., Ko, D. K., Cha, B. H., & Lee, J. (2002). Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases. Journal of Physics D: Applied Physics, 35, 1473–1476. DOI: 10.1088/0022-3727/35/13/303.
Alves, O. L. (1986). Técnicas de síntese em atmosfera inerte. Química Nova, 9, 276–281. (in Portuguese)
Andrade, C. K. Z. (2004). Niobium pentachloride in organic synthesis: Applications and perspectives. Current Organic Synthesis, 1, 333–353.
ASTM (2014). Standard test method for purity by differential scanning calorimetry. West Conshohocken, PA, USA: ASTM International. DOI: 10.1520/e0928-08r14.
Bartolomeu, A. A., Menezes, M. L., & Silva-Filho, L. C. (2014). Efficient one-pot synthesis of 14-aryl-14H-dibenzo [a, j]xanthene derivatives promoted by niobium pentachloride. Chemical Papers, 68, 1593–1600. DOI: 10.2478/s11696-014-0597-8.
Bartolomeu, A. A., Menezes, M. L., & Silva-Filho, L. C. (2015). Chemoselective condensation of β-naphthol, dimethyl malonate, and aromatic aldehydes promoted by niobium pentachloride. Synthetic Communications, 45, 1114–1126. DOI: 10.1080/00397911.2014.999341.
Bekaert, A., Andrieux, J., & Plat, M. (1992). New total synthesis of bikaverin. Tetrahedron Letters, 33, 2805–2806. DOI: 10.1016/s0040-4039(00)78863-0.
Bhowmik, B. B., & Ganguly, P. (2005). Photophysics of xan-thene dyes in surfactant solution. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 61, 1997–2003. DOI: 10.1016/j.saa.2004.07.031.
Cao, Y., Yao, C., Qin, B., & Zhang, H. (2013). Solvent-free synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes catalyzed by recyclable and reusable iron(III) triflate. Research on Chemical Intermediates, 39, 3055–3062. DOI: 10.1007/s11164-012-0818-0.
Chibale, K., Visser, M., van Schalkwyk, D., Smith, P. J., Saravanamuthu, A., & Fairlamb, A. H. (2003). Exploring the potential of xanthene derivatives as trypanothione reductase inhibitors and chloroquine potentiating agents. Tetrahedron, 59, 2289–2296. DOI: 10.1016/s0040-4020(03)00240-0.
Dharma Rao, G. B., Kaushik, M. P., & Halve, A. K. (2012). An efficient synthesis of naphtha[1,2-e]oxazinone and 14-substituted-14H-dibenzo[a,j]xanthene derivatives promoted by zinc oxide nanoparticle under thermal and solvent-free conditions. Tetrahedron Letters, 53, 2741–2744. DOI: 10.1016/j.tetlet.2012.03.085.
D’Souza, D. M., & Müller, T. J. J. (2007). Multi-component syntheses of heterocycles by transition-metal catalysis. Chemical Society Reviews, 36, 1095–1108. DOI: 10.1039/ b608235c.
El-Brashy, A. M., El-Sayed Metwally, M., & El-Sepai, F. A. (2004). Spectrophotometric determination of some fluoroquinolone antibacterials by binary complex formation with xanthene dyes. II Farmaco, 59, 809–817. DOI: 10.1016/j. farmac. 2004.07.001.
Fobane, L., Ndam, E. N., & Mbolo, M. J. (2014). Population structure and natural regeneration of Allanblackia floribunda oliv. (Clusiaceae) in a forest concession of East Cameroon. Journal of Biodiversity and Environmental Sciences, 4, 403–408.
Fuller, R. W., Blunt, J. W., Boswell, J. L., Cardellina, J. H., & Boyd, M. R. (1999). Guttiferone F, the first prenylated ben-zophenone from Allanblackia stuhlmannii. Journal of Natural Products, 62, 130–132. DOI: 10.1021/np9801514.
Hiranrat, A., & Mahabusarakam, W. (2008). New acylphloro-glucinols from the leaves of Rhodomyrtus tomentosa. Tetrahedron, 64, 11193–11197. DOI: 10.1016/j.tet.2008.09.054.
Hiranrat, A., Chitbankluoi, W., Mahabusarakam, W., Limsuwan, S., & Voravuthikunchai, S. P. (2012). A new flavellagic acid derivative and phloroglucinol from Rhodomyrtus tomentosa. Natural Product Research, 26, 1904–1909. DOI: 10.1080/14786419.2011.628666.
Hou, J. T., Gao, J. W., & Zhang, Z. H. (2010a). NbCl5: an efficient catalyst for one-pot synthesis of a-aminophosphonates under solvent-free conditions. Applied Organometallic Chemistry, 25, 47–53. DOI: 10.1002/aoc.1687.
Hou, J. T., Liu, Y. H., & Zhang, Z. H. (2010b). NbCl5 as an efficient catalyst for rapid synthesis of quinoxaline derivatives. Journal of Heterocyclic Chemistry, 47, 703–706. DOI: 10.1002/jhet.388.
Hou, J. T., Chen, H. L., & Zhang, Z. H. (2011a). Rapid and efficient trimethylsilyl protection of hydroxyl groups catalyzed by niobium(V) chloride. Phosphorus, Sulfur, and Silicon and the Related Elements, 186, 88–93. DOI: 10.1080/10426507. 2010.482544.
Hou, J. T., Gao, J. W., & Zhang, Z. H. (2011b). An efficient and convenient protocol for the synthesis of diaminotri-arylmethanes. Monatshefte fur Chemie–Chemical Monthly, 142, 495–499. DOI: 10.1007/s00706-011-0461-2.
Horning, E. C, & Horning, M. G. (1946). Methone derivatives of aldehydes. The Journal of Organic Chemistry, 11, 95–99. DOI: 10.1021/jo01171a014.
Ilangovan, A., Malayappasamy, S., Muralidharan, S., & Maruthamuthu, S. (2011). A highly efficient green synthesis of 1,8-dioxo-octahydroxanthenes. Chemistry Central Journal, 5, 81. DOI: 10.1186/1752-153x-5-81.
Iniyavan, P., Sarveswari, S., & Vijayakumar, V. (2015). Microwave-assisted clean synthesis of xanthenes and chromenes in [bmim][PF6] and their antioxidant studies. Research on Chemical Intermediates, 41, 7413–7426. DOI: 10.1007/ s11164-014-1821-4.
Isambert, N., & Lavilla, R. (2008). Heterocycles as key substrates in multicomponent reactions: The fast lane towards molecular complexity. Chemistry–A European Journal, 14, 8444–8454. DOI: 10.1002/chem.200800473.
John, A., Yadav, P. J. P., & Palaniappan, S. (2006). Clean synthesis of 1,8-dioxo-dodecahydroxanthene derivatives catalyzed by polyaniline-p-toluenesulfonate salt in aqueous media. Journal of Molecular Catalysis A: Chemical, 248, 121–125. DOI: 10.1016/j.molcata.2005.12.017.
Karami, B., Zare, Z., & Eskandari, K. (2013a). Molybdate sulfonic acid: preparation, characterization, and application as an effective and reusable catalyst for octahydroxanthene-1,8-dione synthesis. Chemical Papers, 67, 145–154. DOI: 10.2478/s11696-012-0263-y.
Karami, B., Eskandari, K., Gholipour, S., & Jamshidi, M. (2013b). Facile and rapid synthesis of 9-aryl 1,8-dioxoocta-hydroxanthenes derivatives using tungstate sulfuric acid. Organic Preparations and Procedures International: The New Journal for Organic Synthesis, 45, 220–226. DOI: 10.1080/00304948.2013.764790.
Karami, B., Eskandari, K., Zare, Z., & Gholipour, S. (2014). A new access to 1,8-dioxooctahydroxanthenes using yt-trium(III) nitrate hexahydrate and tin(II) chloride dihydrate as effective and reusable catalysts. Chemistry of Heterocyclic Compounds, 49, 1715–1722. DOI: 10.1007/s10593-014-1423-5.
Kaya, M., Basar, E., & Colak, F. (2011). Synthesis and antimicrobial activity of some bisoctahydroxanthene-1,8-dione derivatives. Medicinal Chemistry Research, 20, 1214–1219. DOI: 10.1007/s00044-010-9459-2.
Knight, C. G., & Stephens, T. (1989). Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles. Biochemical Journal, 258, 683–687. DOI: 10.1042/bj2580683.
Knight, D. W., & Little, P. B. (2001). The first efficient method for the intramolecular trapping of benzynes by phenols: a new approach to xanthenes. Journal of the Chemical Society, 14, 1771–1777. DOI: 10.1039/b103834f.
Lacerda, V., Jr., Dos Santos, D. A., Da Silva-Filho, L. C., Greco, S. J., & dos Santos, R. B. (2012). The growing impact of niobium in organic synthesis and catalysis. Aldrichimica Acta, 45, 19–27.
Li, P., Ma, F., Wang, P., & Zhang, Z. H. (2013). Highly efficient low melting mixture catalyzed synthesis of 1,8-dioxo-dodecahydroxanthene derivatives. Chinese Journal of Chemistry, 31, 757–763. DOI: 10.1002/cjoc.201300152.
Limsuwan, S., Trip, E. N., Kouwen, T. R. H. M., Piersma, S., Hiranrat, A., Mahabusarakam, W., Voravuthikunchai, S. P., van Dijl, J. M., & Kayser, O. (2009). Rhodomyrtone: a new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine, 16, 645–651. DOI: 10.1016/j.phymed.2009.01.010.
Locksley, H. D., & Murray, G. (1971). Extractives from Guttiferae. Part X1X. The isolation and structure of two benzophenones, six xanthones and two biflavonoids from the heartwood of Allanblackia floribunda Oliver. Journal of the Chemical Society C: Organic, 1966–1971. DOI: 10.1039/j39710001332.
Lü, H. Y., Li, J. J., & Zhang, Z. H. (2009). ZrOCl2–8H2O: a highly efficient catalyst for the synthesis of 1,8-dioxo-octahydroxanthene derivatives under solvent-free conditions. Applied Organometallic Chemistry, 23, 165–169. DOI: 10.1002/aoc.1488.
Makino, M., & Fujimoto, Y. (1999). Flavanones from Baeckea frutescens. Phytochemistry, 50, 273–277. DOI: 10.1016/ s0031-9422(98)00534-2.
Maleki, B., Gholizadeh, M., & Sepehr, Z. (2011). 1,3,5-Trichloro-2,4,6-triazinetrion: A versatile heterocycle for the one-pot synthesis of 14-aryl-or alkyl-14H-dibenzo[a,tj]xan-thene, 1,8-dioxooctahydroxanthene and 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives under solvent-free conditions. Bulletin of Korean Chemical Society, 32, 1697–1702. DOI: 10.5012/bkcs.2011.32.5.1697.
Maleki, B., Barzegar, S., Sepehr, Z., Kermanian, M., & Tayebee, R. (2012). A novel polymeric catalyst for the one-pot synthesis of xanthene derivatives under solvent-free conditions. Journal of the Iranian Chemical Society, 9, 757–765. DOI: 10.1007/s13738-012-0092-5.
Napoleon, A. A., & Khan, F. R. N. (2014). Potential anti-tubercular and in vitro anti-inflammatory agents: 9-substi-tuted 1,8-dioxo-octahydroxanthenes through cascade/domino reaction by citric fruit juices. Medicinal Chemistry Research, 23, 4749–4760. DOI: 10.1007/s00044-014-1033-x.
Napoleon, A. A., Khan, F. R. N., Jeong, E. D., & Chung, E. H. (2014). Regioselective synthesis of 3,4,6,7-tetrahydro-3,3-dimethyl-9-phenyl-2H-xanthene-1,8(5H,9H)-diones through ascorbic acid catalyzed three-component domino reaction. Tetrahedron Letters, 55, 5656–5659. DOI: 10.1016/j.tetlet. 2014.08.040.
Nkengfack, A. E., Azebaze, G. A., Vardamides, J. C., Fomum, Z. T., & van Heerden, F. R. (2002). A prenylated xanthone from Allanblackia floribunda. Phytochemistry, 60, 381–384. DOI: 10.1016/s0031-9422(02)00036-5.
Ormond, A. B., & Freeman, H. S. (2013). Dye sensitizers for photodynamic therapy. Materials, 6, 817–840. DOI: 10.3390/ma6030817.
Oshiro, P. B., Lima, P. S. S. G., Menezes, M. L., & Silva-Filho, L. C. (2015). Synthesis of 4H-chromenes promoted by NbCl5 through multicomponent reaction. Tetrahedron Letters, 56, 4476–4479. DOI: 10.1016/j.tetlet.2015.05.099.
Peres, V., & Nagem, T. J. (1997). Trioxygenated naturally occurring xanthones. Phytochemistry, 44, 191–214. DOI: 10.1016/s0031-9422(96)00421-9.
Peres, V., Nagem, T. J., & Oliveira, F. F. (2000). Tetraoxy-genated naturally occurring xanthones. Phytochemistry, 55, 683–710. DOI: 10.1016/s0031-9422(00)00303-4.
Pramanik, A., & Bhar, S. (2012). Alumina-sulfuric acid catalyzed eco-friendly synthesis of xanthenediones. Catalysis Communications, 20, 17–24. DOI: 10.1016/j.catcom.2011.12. 036.
Preetam, A., Prasad, D. K., Sharma, J., & Nath, M. (2015). Facile one-pot synthesis of oxo-xanthenes under microwave irradiation. Current Microwave Chemistry, 2, 15–23. DOI: 10.2174/221333560201150212102647.
Saini, A., Kumar, S., & Sandhu, J. (2006). A new LiBr-catalyzed, facile and efficient method for the synthesis of 14-alkyl or aryl-14H-dibenzo[a,j]xanthenes and tetrahydroben-zo[b]pyrans under solvent-free conventional and microwave heating. Synlett, 2006, 1928–1932. DOI: 10.1055/s-2006-947339.
Shirini, F., Mamaghani, M., & Atghia, S. V. (2013). Use of nanoporous Na+-montmorillonite sulfonic acid (SANM) as an eco-benign, efficient and reusable solid acid catalyst for the one-pot synthesis of 14-aryl-14-H-dibenzo[a,j]xanthenes and 1,8-dioxo-dodecahydroxanthene derivatives. Journal of the Iranian Chemical Society, 10, 415–420. DOI: 10.1007/s 13738-012-0174-4.
Shirini, F., Abedini, M., Seddighi, M., Jolodar, O. G., Safarpoor, M., Langroodi, N., & Zamani, S. (2014). Introduction of a new bi-SO3H ionic liquid based on 2,2’-bipyridine as a novel catalyst for the synthesis of various xanthene derivatives. RSC Advances, 4, 63526–63532. DOI: 10.1039/c4ra12361a.
Shirini, F., Langarudi, M. S. N., Seddighi, M., & Jolodar, O. G. (2015). Bi-SO3H functionalized ionic liquid based on DABCO as a mild and efficient catalyst for the synthesis of 1,8-dioxo-octahydro-xanthene and 5-arylmethylene-pyrimidine-2,4,6-trione derivatives. Research on Chemical Intermediates, 41, 8483–8497. DOI: 10.1007/s11164-014-1905-1.
Sianglum, W., Srimanote, P., Wonglumsom, W., Kittiniyom, K., & Voravuthikunchai, S. P. (2011). Proteome analyses of cellular proteins in methicillin-resistant Staphylococcus aureus treated with rhodomyrtone, a novel antibiotic candidate. PLoS ONE, 6, e16628. DOI: 10.1371/journal.pone.0016628.
Soleimani, E., Khodaei, M. M., & Koshvandi, A. T. K. (2011). The efficient synthesis of 14-alkyl or aryl 14H-dibenzo[a,j]xanthenes catalyzed by bismuth(III) chloride under solvent-free conditions. Chinese Chemical Letters, 22, 927–930. DOI: 10.1016/j.cclet.2011.01.012.
Urinda, S., Kundu, D., Majee, A., & Hajra, A. (2009). Indium triflate-catalyzed one-pot synthesis of 14-alkyl or aryl-14H-dibenzo[a,j]xanthenes in water. Heteroatom Chemistry, 20, 232–234. DOI: 10.1002/hc.20539.
Visutthi, M., Srimanote, P., & Voravuthikunchai, S. P. (2011). Responses in the expression of extracellular proteins in methicillin-resistant Staphylococcus aureus treated with rhodomyrtone. The Microbiological Society of Korea, 49, 956–964. DOI: 10.1007/s12275-011-1115-0.
Wang, J. Q., & Harvey, R. G. (2002). Synthesis of polycyclic xanthenes and furans via palladium-catalyzed cyclization of polycyclic aryltriflate esters. Tetrahedron, 58, 5927–5931. DOI: 10.1016/s0040-4020(02)00534-3.
Wang, L. M., Sui, Y. Y., & Zhang, L. (2008). Synthesis of 14-[(un)substituted phenyl] or alkyl-14H-dibenzo[a,j]xanthenes using Yb(OTf)3 as an efficient catalyst under solvent-free conditions. Chinese Journal of Chemistry, 26, 1105–1108. DOI: 10.1002/cjoc. 200890196.
Zhang, Z. H., & Liu, Y. H. (2008). Antimony trichloride/SiO2 promoted synthesis of 9-ary-3,4,5,6,7,9-hexahydroxanthene-1,8-diones. Catalysis Communications, 9, 1715–1719. DOI: 10.1016/j.catcom.2008.01.031.
Zhang, Z., & Tao, X. (2008). 2,4,6-Trichloro-1,3,5-triazine-promoted synthesis of 1,8-dioxo-octahydroxanthenes under solvent-free conditions. Australian Journal of Chemistry, 61, 77–79. DOI: 10.1071/ch07274.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
dos Santos, W.H., Da Silva-Filho, L.C. Facile and efficient synthesis of xanthenedione derivatives promoted by niobium pentachloride. Chem. Pap. 70, 1658–1664 (2016). https://doi.org/10.1515/chempap-2016-0098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2016-0098