Abstract
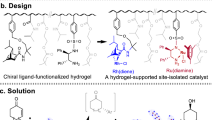
Chiral products play an important role particularly in the field of medicinal chemistry, where it is known that enantiomers often have very different biological properties and effects. One of the most powerful tool to obtain a product as a single enantiomer is asymmetric catalysis. Recently, organocatalysis, i.e. the use of small organic molecules to catalyze enantioselective transformations, has emerged as a prominent field in asymmetric synthesis. In this work, the use of hydrogels as a support for a chiral imidazolidinone organocatalyst (MacMillan catalyst) and its application in the reduction of activated olefins mediated by the Hantzsch ester is reported for the first time. Results showed a good activity of hydrogels in respect to both yield and enantioselection.
Similar content being viewed by others
References
Annabi, N., Tamayol, A., Uquillas, J. A., Akbari, M., Bertassoni, L. E., Cha, C., Camci-Unal, G., Dokmeci, M. R., Peppas, N. A., & Khademhosseini, A. (2014). 25th Anniversary article: Rational design and applications of hydrogels in regenerative medicine. Advanced Materials, 26, 85–124. DOI: 10.1002/adma.201303233.
Atodiresei, I., Vila, C., & Rueping, M. (2015). Asymmetric organocatalysis in continuous flow: Opportunities for impacting industrial catalysis. ACS Catalysis, 5, 1972–1985. DOI: 10.1021/acscatal.5b00002.
Blackmond, D. G., Armstrong, A., Coombe, V., & Wells, A. (2007). Water in organocatalytic processes: Debunking the myths. Angewandte Chemie International Edition, 46, 3798–3800. DOI: 10.1002/anie.200604952.
Brenna, E., Gatti, F. G., Manfredi, A., Monti, D., & Parmeggiani, F. (2012a). Enoate reductase-mediated preparation of methyl (S)-2-bromobutanoate, a useful key intermediate for the synthesis of chiral active pharmaceutical ingredients. Organic Process Research & Development, 16, 262–268. DOI: 10.1021/op200086t.
Brenna, E., Gatti, F. G., Monti, D., Parmeggiani, F., & Sacchetti, A. (2012b). Cascade coupling of ene reductases with alcohol dehydrogenases: Enantioselective reduction of prochiral unsaturated aldehydes. ChemCatChem, 4, 653–659. DOI: 10.1002/cctc.201100418.
Brenna, E., Gatti, F. G., Monti, D., Parmeggiani, F., & Sacchetti, A. (2012c). Productivity enhancement of C=C bioreductions by coupling the in situ substrate feeding product removal technology with isolated enzymes. Chemical Communications, 48, 79–81. DOI: 10.1039/c1cc16014a.
Brenna, E., Cosi, S. L., Ferrandi, E. E., Gatti, F. G., Monti, D., Parmeggiani, F., & Sacchetti, A. (2013a). Substrate scope and synthetic applications of the enantioselective reduction of a-alkyl-β-arylenones mediated by Old Yellow Enzymes. Organic & Biomolecular Chemistry, 11, 2988–2996. DOI: 10.1039/c3ob40076j.
Brenna, E., Gatti, F. G., Malpezzi, L., Monti, D., Parmeggiani, F., & Sacchetti, A. (2013b). Synthesis of robalzotan, ebalzotan, and rotigotine precursors via the stereoselective multienzymatic cascade reduction of α, β-unsaturated aldehydes. The Journal of Organic Chemistry, 78, 4811–4822. DOI: 10.1021/jo4003097.
Brenna, E., Gatti, F. G., Monti, D., Parmeggiani, F., Sacchetti, A., & Valoti, J. (2015). Substrate-engineering approach to the stereoselective chemo-multienzymatic cascade synthesis of Nicotiana tabacum lactone. Journal of Molecular Catalysis B: Enzymatic, 114, 77–85. DOI: 10.1016/j.molcatb.2014. 12.011.
Dalko, P. I., & Moisan, L. (2001). Enantioselective organocatalysis. Angewandte Chemie International Edition, 40, 3726–3748. DOI: 10.1002/1521–3773(20011015)40:20<3726::aidanie3726>3.0.co;2-d.
Dalko, P. I., & Moisan, L. (2004). In the golden age of organocatalysis. Angewandte Chemie International Edition, 43, 5138–5175. DOI: 10.1002/anie.200400650.
Danelli, T., Annunziata, R., Benaglia, M., Cinquini, M., Cozzi, F., & Tocco, G. (2003). Immobilization of catalysts derived from Cinchona alkaloids on modified poly(ethylene glycol). Tetrahedron: Asymmetry, 14, 461–467. DOI: 10.1016/s09574166(02)00830-3.
Davoodnia, A., Allameh, S., Fazli, S., & Tavakoli-Hoseini, N. (2011). One-pot synthesis of 2-amino-3-cyano-4-arylsubstituted tetrahydrobenzo[b]pyrans catalysed by silica gel-supported polyphosphoric acid (PPA-SiO2) as an efficient and reusable catalyst. Chemical Papers, 65, 714–720. DOI: 10.2478/s11696-011-0064–8.
Dondoni, A., & Massi, A. (2008). Asymmetric organocatalysis: From infancy to adolescence. Angewandte Chemie International Edition, 47, 4638–4660. DOI: 10.1002/anie.200704684.
Ford, M. C., Bertram, J. P., Hynes, S. R., Michaud, M., Li, Q., Young, M., Segal, S. S., Madri, J. A., & Lavik, E. B. (2006). A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proceedings of the National Academy of Sciences of the United States of America, 103, 2512–2517. DOI: 10.1073/pnas.0506020102.
Giacalone, F., Gruttadauria, M., Agrigento, P., & Noto, R. (2012). Low-loading asymmetric organocatalysis. Chemical Society Reviews, 41, 2406–2447. DOI: 10.1039/c1cs15206h.
Hayashi, Y. (2006). In water or in the presence of water? Angewandte Chemie International Edition, 45, 8103–8104. DOI: 10.1002/anie.200603378.
Hiki, S., & Kataoka, K. (2007). A facile synthesis of azidoterminated heterobifunctional poly(ethylene glycol)s for “click” conjugation. Bioconjugate Chemistry, 18, 2191–2196. DOI: 10.1021/bc700152j.
Itsuno, S., & Hassan, M. M. (2014). Polymer-immobilized chiral catalysts. RSC Advances, 4, 52023–52043. DOI: 10.1039/c4ra09561h.
Kolb, H. C., Finn, M. G., & Sharpless, K. B. (2001). Clickchemistry: Diverse chemical function from a few good reactions. Angewandte Chemie International Edition, 40, 2004–2021. DOI: 10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.0.co;2-5.
Lelais, G., & MacMillan, D. W. C. (2006). Modern strategies in organic catalysis: The advent and development of iminium activation. Aldrichimica Acta, 39, 79–87.
MacMillan, D. W. C. (2008). The advent and development of organocatalysis. Nature, 455, 304–308. DOI: 10.1038/nature07367.
Moses, J. E., & Moorhouse, A. D. (2007). The growing applications of click chemistry. Chemical Society Reviews, 36, 12491262. DOI: 10.1039/b613014n.
Munirathinam, R., Huskens, J., & Verboom, W. (2015). Supported catalysis in continuous-flow microreactors. Advanced Synthesis & Catalysis, 357, 1093–1123. DOI: 10.1002/adsc. 201401081.
Ouellet, S. G., Tuttle, J. B., & MacMillan, D. W. C. (2005). Enantioselective organocatalytic hydride reduction. Journal of the American Chemical Society, 127, 32–33. DOI: 10.1021/ja043834g.
Ouellet, S. G., Walji, A. M., & MacMillan, D. W. C. (2007). Enantioselective organocatalytic transfer hydrogenation reactions using Hantzsch esters. Accounts of Chemical Research, 40, 1327–1339. DOI: 10.1021/ar7001864.
Park, S. Y., Lee, J. W., & Song, C. E. (2015). Parts-permillion level loading organocatalysed enantioselective silylation of alcohols. Nature Communications, 6, 7512, DOI: 10.1038/ncomms8512.
Puglisi, A., Benaglia, M., Cinquini, M., Cozzi, F., & Celentano, G. (2004). Enantioselective 1,3-dipolar cycloadditions of unsaturated aldehydes promoted by a poly(ethylene glycolsupported organic catalyst. European Journal of Organic Chemistry, 2004, 567–573. DOI: 10.1002/ejoc.200300571.
Rossi, F., Perale, G., Storti, G., & Masi, M. (2012). A library of tunable agarose carbomer-based hydrogels for tissue engineering applications: The role of cross-linkers. Journal of Applied Polymer Science, 123, 2211–2221. DOI: 10.1002/app.34731.
Sacchetti, A., Mauri, E., Sani, M., Masi, M., & Rossi, F. (2014). Microwave-assisted synthesis and click chemistry as simple and efficient strategy for RGD functionalized hydrogels. Tetrahedron Letters, 55, 6817–6820. DOI: 10.1016/j.tetlet.2014.10.069.
Santoro, M., Marchetti, P., Rossi, F., Perale, G., Castiglione, F., Mele, A., & Masi, M. (2011). Smart approach to evaluate drug diffusivity in injectable agar-carbomer hydrogels for drug delivery. The Journal of Physical Chemistry B, 115, 2503–2510. DOI: 10.1021/jp1111394.
Seayad, J., & List, B. (2005). Asymmetric organocatalysis. Organic & Biomolecular Chemistry, 3, 719–724. DOI: 10.1039/b415217b.
Shi, J. Y., Wang, C. A., Li, Z. J., Wang, Q., Zhang, Y., & Wang, W. (2011). Heterogeneous organocatalysis at work: Functionalization of hollow periodic mesoporous organosilica spheres with MacMillan catalyst. Chemistry A European Journal, 17, 6206–6213. DOI: 10.1002/chem.201100072.
Slaughter, B. V., Khurshid, S. S., Fisher, O. Z., Khademhosseini, A., & Peppas, N. A. (2009). Hydrogels in regenerative medicine. Advanced Materials, 21, 3307–3329. DOI: 10.1002/adma.200802106.
Zhu, J., Tang, C., Kottke-Marchant, K., & Marchant, R. E. (2009). Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjugate Chemistry, 20, 333–339. DOI: 10.1021/bc800441v.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sacchetti, A., Rossi, F., Rossetti, A. et al. Hydrogel supported chiral imidazolidinone for organocatalytic enantioselective reduction of olefins in water. Chem. Pap. 70, 436–444 (2016). https://doi.org/10.1515/chempap-2015-0232
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0232