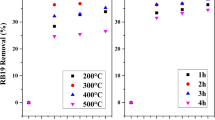
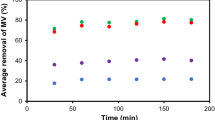
Abstract
This comparative study focused on the different methods used for the treatment of model waste water polluted with the chlorinated acid dye Mordant Blue 9. Low-cost and commercially available ionic liquids — benzalkonium chloride and Aliquat 336 — were applied as liquid ion-exchangers to precipitate the Mordant Blue 9 by way of ion pair formation between the bulky ammonium cations of ionic liquids and anions of the above dye. The decolorisation efficiency of the ionic liquid application and the effect on reduction of the absorbance, adsorbable organic halogens, chemical oxygen demand and biological oxygen demand were compared with the conventional coagulation, sorption and Fenton oxidation techniques.
Similar content being viewed by others
References
Aksu, Z., Ertugrul, S., & Donmez, G. (2009). Single and binary chromium(VI) and Remazol Black B biosorption properties of Phormidium sp. Journal of Hazardous Materials, 168, 310–318. DOI: 10.1016/j.jhazmat.2009.02.027.
Álvarez, M. S., Moscoso, F., Rodriguez, A., Sanroman, M. A., & Deive, F. J. (2013). Novel physico-biological treatment for the remediation of textile dyes-containing industrial effluents. Bioresource Technology, 146, 689–695. DOI: 10.1016/j.biortech.2013.07.137.
Borodkin, V. F. (1987). Chemistry of organic dyes. Prague, Czech Republic: SNTL.
British Standards Institution (1998). Water quality. Determination of biochemical oxygen demand after n days (BODn). Dilution and seeding method with allylthiourea addition. BSEN 1899-1:1998, BS 6068-2.63:1998. London, UK: British Standards Institution.
Chun, S. K., Dzyuba, S. V., & Bartsch, R. A. (2001). Influence of sturctural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether. Analytical Chemistry, 73, 3737–3741. DOI: 10.1021/ac010061v.
Czech Standards Institute (1989). Water quality — Determination of the chemical oxygen demand. CSN ISO 6060. Praha, Czech Republic: Czech Standards Institute.
Freemantle, M. (1998). Designer solvents. Ionic liquids may boost clean technology development. Chemical & Engineering News, 76(13), 32–37. DOI: 10.1021/cen-v076n013.p032.
Gordon, P. F., & Gregory, P. (1983). Azo dyes. In P. F. Gordon, & P. Gregory (Eds.), Organic chemistry in colour (pp. 95–162). Berlin, Germany: Springer. DOI: 10.1007/978-3-642-82959-8.
Koprivanac, N., Kusic, H., Vujevic, D., Peternel, I., & Locke, B. R. (2005). Influence of iron on degradation of organic dyes in corona. Journal of Hazardous Materials, 117, 113–119. DOI: 10.1016/j.jhazmat.2004.03.023.
Meindersma, G., Maase, M., & De Haan, A. (2007). Ullmann’s encyklopedia of industrial chemistry. Weinheim, Germany: Wiley.
Mikulasek, P., & Cuhorka, J. (2010). Nanofiltration in the manufacture of liquid dyes production. Water Science & Technology, 61, 1865–1873. DOI: 10.2166/wst.2010.871.
Namasivayam, C., & Kavitha, D. (2002). Removal of Congo Red from water by adsorption onto activated carbon pre-pared from coir pith, an agricultural solid waste. Dyes and Pigments, 54, 47–58. DOI: 10.1016/s0143-7208(02)00025-6.
Natarajan, T. S., Bajaj, H. C., & Tayade, R. J. (2014). Preferential adsorption behavior of methylene blue dye onto surface hydroxyl group enriched TiO2 nanotube and its photocatalytic regeneration. Journal of Colloid and Interface Scince, 433, 104–114. DOI: 10.1016/j.jcis.2014.07.019.
Pakshirajan, K., & Singh, S. (2010). Decolorization of synthetic wastewater containing azo dyes in a batch-operated rotating biological contactor reactor with the immobilized fungus Phanerochaete chrysosporium. Industrial & Engineering Chemistry Research, 49, 7484–7487. DOI: 10.1021/ie1007079.
Seddon, K. R. (1997). Ionic liquids for clean technology. Journal of Chemical Technology & Biotechnology, 68, 351–356. DOI: 10.1002/(sici)1097-4660(199704)68:4<351::aid-jctb613>3.0.co;2–4.
Sun, X. Q., Ji, Y., Guo, L., Chen, J., & Li, D. Q. (2011). A novel ammonium ionic liquid based extraction strategy for separating scandium from yttrium and lanthanides. Separation and Purification Technology, 81, 25–30. DOI: 10.1016/j.seppur.2011.06.034.
Tayade, R. J., Natarajan, T. S., Bajaj, H. C. (2009). Photocatalytic degradation of methylene blue using ultraviolet light emitting diodes. Industrial & Engineering Chemistry Research, 48, 10262–10267. DOI: 10.1021/ie9012437.
Wawrzkiewicz, M. (2012). Anion exchange resins as effective sorbents for acidic dye removal from aqueous solutions and wastewaters. Solvent Extraction and Ion Exchange, 30, 507–523. DOI: 10.1080/07366299.2011.639253.
Weidlich, T., & Martinkova, J. (2012). Application of tetraphenyl- and ethylriphenylphosphonium salts for separation of reactive dyes from aqueous solution. Separation Science and Technology, 47, 1310–1315. DOI: 10.1080/01496395.2012.672527.
Weidlich, T., & Martínková, J. (2013). CZ Patent: CZ201203 59A. Praha, Czech Republic: Úřad průmyslového vlastnictví.
Weidlich, T., Prokeš, L., & Pospíšilová, D. (2013). Debromination of 2,4,6-tribromophenol coupled with biodegradation. Central European Journal of Chemistry, 11, 979–987. DOI: 10.2478/s11532-013-0231-6.
Weidlich, T., Opršal, J., Krejčová, A., & Jašúrek, B. (2015). Effect of glucose on lowering Al-Ni alloy consumption in dehalogenation of halogenoanilines. Monatshefte für Chemie — Chemical Monthly, 146, 613–620. DOI: 10.1007/s00706-014-1344-0.
World Dye Variety (2015). Mordant Blue 9. Retrieved April 10, 2015, from http://www.worlddyevariety.com/mordantdyes/mordant-blue-9.html
Zhu, M. G., Zhao, J. M., Li, Y. B., Mehio, N., Qi, Y. R., Liu, H. Z., & Dai, S. (2015). An ionic liquid-based synergistic extraction strategy for rare earths. Green Chemistry, 17, 2981–2993. DOI: 10.1039/c5gc00360a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 42nd International Conference of the Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 25–29 May 2015.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Šimek, M., Mikulášek, P., Kalenda, P. et al. Possibilities for removal of chlorinated dye Mordant Blue 9 from model waste water. Chem. Pap. 70, 470–476 (2016). https://doi.org/10.1515/chempap-2015-0225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0225