Abstract
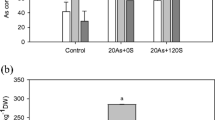
Food-based exposure to arsenic is a human carcinogen and can severely impact human health resulting in many cancerous diseases and various neurological and vascular disorders. This project is a part of our attempts to develop new varieties of crops for avoiding arsenic contaminated foods. For this purpose, we have previously identified four key genes, and molecular functions of two of these, AtACR2 and AtPCS1, have been studied based on both in silico and in vivo experiments. In the present study, a T-DNA tagged mutant, (SALK_143282C with mutation in AtACR2 gene) of Arabidopsis thaliana was studied for further verification of the function of AtACR2 gene. Semi-quantitative RT-PCR analyses revealed that this mutant exhibits a significantly reduced expression of the AtACR2 gene. When exposed to 100 μM of arsenate (AsV) for three weeks, the mutant plants accumulated arsenic approximately three times higher (778 μg/g d. wt.) than that observed in the control plants (235 μg/g d. wt.). In contrast, when the plants were exposed to 100 j.M of arsenite (AsIII), no significant difference in arsenic accumulation was observed between the control and the mutant plants (535 μg/g d. wt. and 498 μg/g d. wt., respectively). Also, when arsenate and arsenite was measured separately either in shoots or roots, significant differences in accumulation of these substances were observed between the mutant and the control plants. These results suggest that AtACR2 gene is involved not only in accumulation of arsenic in plants, but also in conversion of arsenate to arsenite inside the plant cells.
Similar content being viewed by others
Abbreviations
- ACR2:
-
arsenate reductase 2
- As:
-
arsenic
- AsIII:
-
arsenite
- AsV:
-
arsenate
- DMA:
-
dimethylarsinic acid
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- Gpase:
-
glycogen phosphorylase
- Grx:
-
glutaredoxin
- GSH:
-
glutathione
- ICP-DRC-MS:
-
inductively coupled plasma dynamic reaction cell mass spectrometry
- ICV:
-
initial calibration verification
- MMA:
-
monomethylarsonic acid
- MS:
-
Murashige-Skoog
- MT:
-
mutant type
- PNPase:
-
purine nucleoside phosphorylase
- PT-Pase:
-
protein tyrosine phosphatase
- WT:
-
wild type
References
Ali W., Isayenkov S.V., Zhao F.J. & Maathuis F.J. 2009. Arsenite transport in plants. Cell. Mol. Life Sci. 66: 2329–2339.
Bhattacharjee H. & Rosen B.P. 2007. Arsenic metabolism in prokaryotic and eukaryotic microbes, pp. 371–406. In: Nies D.H. & Silver S. (eds) Molecular Microbiology of Heavy Metals. Springer-Verlag, Berlin, Germany.
Bleeker P.M., Hakvoort H.W.J., Bliek M., Souer E. & Schat H. 2006. Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate tolerant Holcus lanatus. Plant J. 45: 917–929.
Blum R., Beck A., Korte A., Stengel A., Letzel T., Lendzian K. & Grill E. 2007. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 49: 740–749.
Bundschuh J., Nath B., Bhattacharya P., Liu C.W., Armienta M.A., Moreno Lopez M.V., Lopez D.L., Jean J.S., Cornejo L., Lauer Macedo L.F. & Filho A.T. 2012. Arsenic in the human food chain: the Latin American perspective. Sci. Total Environ. 429: 92–106.
Cobbett C.S. 2000. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 123: 825–832.
Dhankher O.P., Rosen B.P., McKinney E.C. & Meagher R.B. 2006. Hyperaccumulation of arsenic in the shoots of Ara-bidopsis silenced for arsenate reductase (ACR2). Proc. Natl. Acad. Sci. USA 103: 5413–5418.
Duan G.L., Zhou Y., Tong Y.P., Mukhopadhyay R., Rosen B.P. & Zhu Y.G. 2007. A CDC25 homologue from rice functions as an arsenate reductase. New Phytologist 174: 311–321.
Elke M., Lorentzen L. & Kingston H.M. 1996. Comparison of microwave-assisted and conventional leaching using EPA method 3050B. Anal. Chem. 68: 4316–4320.
Ellis D.R., Gumaelius L., Indriolo E., Pickering I. J., Banks J.A. & Salt D.E. 2006. A novel arsenate reductase from the arsenic hyperaccumulating fern Pteris vittata. Plant Physiol. 141: 1544–1554.
Gregus Z. & Nemeti B. 2002. Purine nucleoside phosphorylase as a cytosolic arsenate reductase. Toxicol. Sci. 70: 13–19.
Gregus Z. & Nemeti B. 2005. The glycolytic enzyme glyceralde-hyde-3-phosphate dehydrogenase works as an arsenate reductase in human red blood cells and rat liver cytosol. Toxicol. Sci. 85: 859–869.
Gregus Z. & Nemeti B. 2007. Glutathione-dependent reduction of arsenate by glycogen phosphorylase responsiveness to endogenous and xenobiotic inhibitors. Toxicol. Sci. 100: 44–53.
Halder D., Bhowmick S., Biswas A., Mandal U., Nriagu J., Guha Mazumder D.N., Chatterjee D. & Bhattacharya P. 2012. Consumption of brown rice: a potential pathway for arsenic exposure in rural Bengal. Environ. Sci. Technol. 46: 4142–4148.
Koch I., Wang L., Ollson C, Cullen W.R. & Reimer K.J. 2000. The predominance of inorganic arsenic species in plants from Yellowknife, Northwest Territories, Canada. Environ. Sci. Technol. 34: 22–26.
Landrieu L, da Costa M., De Veylder L., Dewitte F., Vandepoele K., Hassan S., Wieruszeski J.M., Corellou F., Faure J.D., Van Montagu M., Inze D. & Lippens G. 2004. A small CDC25 dual-specificity tyrosine-phosphatase isoform in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 101: 13380–13385.
Liu W., Schat H., Bliek M., Chen Y., McGrath S.P., George G., Salt D.E. & Zhao F.J. 2012. Knocking out ACR2 does not affect arsenic redox status in Arabidopsis thaliana: implications for As detoxification and accumulation in plants. PLoS One 7: e42408.
Lombi E., Zhao F.J., Fuhrmann M., Ma L.Q. & McGrath S.P. 2002. Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol. 156: 195–203.
Lund D., Larsson D., Nahar N. & Mandal A. 2010. Arsenic accumulation in plants - outlining strategies for developing improved variety of crops for avoiding arsenic toxicity in foods. J. Biol. Sys. 18: 223–224.
Lutsenko S. & Arguello J.M. 2012. Metal Transporters: Current Topics in Membranes. Academic Press, Waltham, USA, 328 pp. ISBN 978-0-12-394390-3.
Meharg A.A. & Hartley-Whitaker J. 2002. Arsenic uptake and metabolism in arsenic resistant and non-resistant plant species. New Phytol. 154: 29–43.
Messens J. & Silver S. 2006. Arsenate reduction: thiol cascade chemistry with convergent evolution. J. Mol. Biol. 362: 1–17.
Mukhopadhyay R., Shi J. & Rosen B.P. 2000. Purification and characterization of ACR2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 275: 21149–21157.
Murashige T. & Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 15: 473–497.
Nahar N., Rahman A., Mos M., Warzecha T., Algerin M., Ghosh S., Johnson-Brousseau S. & Mandal A. 2012. In silico and in vivo studies of an Arabidopsis thaliana gene ACR2 putatively involved in arsenic accumulation in plants. J. Mol. Model. 18: 4249–4262.
Neidhardt H., Norra S., Tang X., Guo H. & Stuben D. 2012. Impact of irrigation with high arsenic burden groundwater on the soil-plant system: result from a case study in the Inner Mongolia, China. Environ. Poll. 163: 8–13.
Rahman A., Nahar N., Nawani N.N., Jass J., Desale P., Kapadnis B.P., Hossain K., Saha A.K., Ghosh S., Olsson B. & Mandal A. 2014. Isolation of a Lysinibacillus strain Bl-CDA showing potentials for arsenic bioremediation. J. Environ. Sci. Health, Part A. 49: 1349–1360.
Rahman A., Nahar N., Nawani N.N., Jass J., Ghosh S., Olsson B. & Mandal A. 2015. Comparative genome analysis of Lysinibacillus Bl-CDA, a bacterium that accumulates arsenics. Genomics 106: 384–392.
Tripathi R.D., Srivastava S., Mishra S., Singh N., Tuli R., Gupta D.K. Maathuis F.J.M. 2007. Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol. 25: 158–165.
Van den Broeck K., Vandecasteele C. & Geuns J.M.C. 1998. Speciation by liquid chromatography-inductively coupled plasma-mass spectrometry of arsenic in mung bean seedlings used as a bio-indicator for arsenic contamination. Anal. Chim. Acta 361: 101–111.
Webb S.M., Gaillard J.F., Ma L., Tu C. 2003. XAS speciation of arsenic in a hyper-accumulating fern. Environ. Sci. Technol. 37: 754–760.
Wood S.A., Tait CD. & Janecky D.R. 2002. A Raman spectroscopic study of arsenite and thioarsenite species in aqueous solution at 25 °C. Geochem. Trans. 3: 31.
Zhou Y., Messier N., Ouellette M., Rosen B.P. & Mukhopadhyay R. 2004. Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug Pen-tostam. J. Biol. Chem. 279: 37445–37451.
Zhao F.J, Ma J.F, Meharg A.A, McGrath S.P. 2009 Arsenic uptake and metabolism in plants. New Phytol. 181: 777–794.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nahar, N., Rahman, A., Ghosh, S. et al. Functional studies of AtACR2 gene putatively involved in accumulation, reduction and/or sequestration of arsenic species in plants. Biologia 72, 520–526 (2017). https://doi.org/10.1515/biolog-2017-0062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2017-0062