Abstract
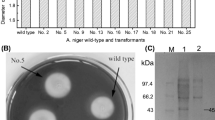
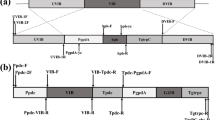
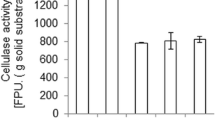
A plasmid pCAMBIA1301 containing Pleurotus eryngii cellulase gene (PEcbh), under the control of Lentinus edodes glyceraldehyde-3-phosphate dehydrogenase (LEgpd) promoter, was constructed and used as an expression vector. The vector was transformed into the tissue of P. eryngii using Agrobacterium tumefaciens-mediated transformation (ATMT) method and 4 transformants (PET1-4) were obtained. The positive transformants were confirmed by cultivation in media containing hygromycin and by PCR amplification of hygromycin B resistance-LEgpd promoter gene fragment. Unpredicted, cellulase specific activities of the transformants were not higher than those of the wild type. Mushroom cultivation was performed in the laboratory and the results revealed that the average biological efficiency of PET4 was significantly 1.52 times higher than those of the wild type.
Similar content being viewed by others
Abbreviations
- ATMT:
-
Agrobacterium tumefaciens-mediated transformation
- BE:
-
biological efficiency
- CMC:
-
carboxymethylcellulose
- gus:
-
β-glucuronidase
- hph:
-
hygromycin B resistance
- LEgpd:
-
Lentinus edodes glyceraldehyde-3-phosphate dehydrogenase
- PDA:
-
potato dextrose agar
- PEcbh:
-
Pleurotus eryngii cellulase gene
- PET1-4:
-
Pleurotus eryngii transformant strains 1-4
References
Chen S.Y., Ho K.J., Hsieh Y.J., Wang L.T. & Mau J.L. 2012. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT Food Sci. Technol. 47: 274–278.
Chen X., Stone M., Schlagnhaufer C. & Romaine C.P. 2000. A fruiting body tissue method for efficient Agrobacterium-mediated transformation of Agaricus bisporus. Appl. Environ. Microbiol. 66: 4510–4513.
Cho J.H., Lee S.E., ChangW.B. & Cha J.S. 2006. Agrobacterium-mediated transformation of the winter mushroom, Flammulina velutipes. Korean J. Mycol. 34: 104–107.
Chukeatirote E., Maharachchikumbura S.S.N., Wongkham S., Sysouphanthong P., Phookamsak R. & Hyde K.D. 2012. Cloning and sequence analysis of the cellobiohydrolase I genes from some basidiomycetes. Korean J. Mycol. 40: 107–110.
Febriki-Ourang S., Jalali-Javaran M., Mohammadi-Goltapeh E., Alizadeh H. & Honari H. 2013. Optimization of Agrobacterium-mediated transformation in oyster mushroom (Pleurotus ostreatus) by vector containing human pro-insulin gene. Iranian J. Genet. Plant Breed. 2: 1–9.
Irwin D., Shin D.H., Zhang S., Barr B.K., Sakon J., Karplus P.A. & Wilson D.B. 1998. Roles of the catalytic domain and two cellulose binding domains of Thermomonospora fusca E4 in cellulose hydrolysis. J. Bacteriol. 180: 1709–1714.
Kim S., Sapkota K., Choi B.S. & Kim S.J. 2010. Expression of human growth hormone gene in Pleurotus eryngii. Cent. Eur. J. Biol. 5: 791–799.
Kurt S. & Buyukalaca S. 2010. Yield performances and changes in enzyme activities of Pleurotus spp. (P. ostreatus and P. sajorcaju) cultivated on different agricultural wastes. Bioresour. Technol. 101: 3164–3169.
Lee C.C., Wong D.W.S. & Robertson G.H. 2001. Cloning and characterization of two cellulase genes from Lentinula edodes. FEMS Microbiol. Lett. 205: 355–360.
Michielse C.B., Hooykaas P.J.J., Hondel C.A.M.J.J. & Ram A.F.J. 2005. Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Curr. Genet. 48: 1–17.
Michielse C.B., Hooykaas P.J.J., Hondel C.A.M.J.J. & Ram A.F.J. 2008. Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat. Protoc. 3: 1671–1678.
Mikosch T.S.P., Lavrijssen B., Sonnenberg A.S.M. & Griensven L.J.L.D. 2001. Transformation of the cultivated mushroom Agaricus bisporus (Lange) using T-DNA from Agrobacterium tumefaciens. Curr. Genet. 39: 35–39.
Moonmoon M., Uddin M.N., Ahmed S., Shelly N.J. & Khan M.A. 2010. Cultivation of different strains of king oyster mushroom (Pleurotus eryngii) on saw dust and rice straw in Bangladesh. Saudi J. Biol. Sci. 17: 341–345.
Okamoto T., Yamada M., Sekiya S., Okuhara T., Taguchi G., Inatomi S. & Shimosaka M. 2010. Agrobacterium tumefaciens-mediated transformation of the vegetative dikaryotic mycelium of the cultivated mushroom Flammulina velutipes. Biosci. Biotechnol. Biochem. 74: 2327–2329.
Rodriguez Estrada A.E., Jimenez-Gasco M.M. & Royse D.J. 2009. Improvement of yield of Pleurotus eryngii var. eryngii by substrate supplementation and use of a casing overlay. Bioresour. Technol. 100: 5270–5276.
Rodriguez Estrada A.E. & Royse D.J. 2007. Yield, size and bacterial blotch resistance of Pleurotus eryngii grown on cottonseed hulls/oak sawdust supplemented with manganese, copper and whole ground soybean. Bioresour. Technol. 98: 1898–1906.
Romruen U. & Bangyeekhun E. 2016. Cloning and expression of the cellulase gene from the king oyster mushroom, Pleurotus eryngii. Silpakorn U. Sci. Technol. J. 10: 22–30.
Stamets P. 1993. Growing Gourmet and Medicinal Mushrooms. Ten Speed Press, Berkeley. 574 pp.
Wang H. & Ng T.B. 2004. Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii. Peptides 25: 1–5.
Wang J., Guo L., Zhang K., Wu Q. & Lin J. 2008. Highly efficient Agrobacterium-mediated transformation of Volvariella volvacea. Bioresour. Technol. 99: 8524–8527.
Weigel D. & Glazebrook J. 2006. Transformation of Agrobacterium using the freeze-thaw method. CSH Protoc. 7: 3.
Zhao F.Y., Lin J.F., Zeng X.L., Guo L.Q., Wang Y.H. & You L.R. 2010. Improvement in fruiting body yield by introduction of the Ampullaria crossean multi-functional cellulase gene into Volvariella volvacea. Bioresour. Technol. 101: 6482–6486.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romruen, U., Bangyeekhun, E. Yield improvement of the king oyster mushroom, Pleurotus eryngii, by transformation of its cellulase gene. Biologia 72, 140–144 (2017). https://doi.org/10.1515/biolog-2017-0023
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2017-0023