Abstract
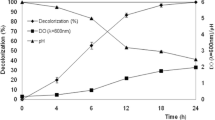
Saccharomyces cerevisiae was able to degrade a highly toxic textile dye malachite green (MG) at 100 mg/L concentration. Although 99% decolourization was observed, a tremendous metabolic and oxidative stress was exerted on the cells. Ethanolic extracts of Terminalia chebula, Clitoria ternatea and Boerhaavia diffusa at a concentration of 1 mg/mL were independently supplied to S. cerevisiae cells to counter the stress. T. chebula, C. ternatea and B. diffusa extracts reduced the activities of glutathione peroxidase (67, 8 and 71%), superoxide dismutase (2, 7 and 16%) and catalase (16, 52 and 57%), respectively. Inductions in the activities of laccase (66, 82 and 50%), lignin peroxidase (35, 75 and 10%), NADH-DCIP reductase (43, 52 and 91%) and MG reductase (66, 126 and 117%) were observed respectively. Presence of dye (MG) extended the lag phase of the growth cycle of S. cerevisiae up to 36 h, which was observed to be restored to normal (4 h) after phytoextract supplementation. Scanning electron microscope imaging revealed the restored cell morphology upon exposure to plant extracts. The accumulation of reactive oxygen species (ROS) was observed to be 355% greater in cells exposed to MG, which was significantly reduced after phytoextracts augmentation when compared to control cells. Phytoextracts proved to be beneficial in increasing the viability of S. cerevisiae cells and reduced the intracellular ROS and nuclear damage. Inclusion of plant extracts during decolourization proved to be beneficial and protected the cells so that 20 treatment cycles could be run achieving significant removal of MG.
Similar content being viewed by others
Abbreviations
- ABTS:
-
2,2-azino-bis-3-ethyl benzothiazoline-6-sulphonic acid
- a.u.:
-
arbitrary unit
- CFUs:
-
colony forming units
- DAPI:
-
4’,6-diamidino-2-phenylindole
- H2DCF:
-
2’7’-dichlorofluorescin
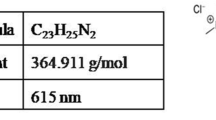
- MG:
-
malachite green
- ROS:
-
reactive oxygen species
- SEM:
-
scanning electron microscope
References
Allen S.A., Clark W., McCaffery J.M., Cai Z., Lanctot A., Slininger P.J., Liu Z.L. & Gorsich S.W. 2010. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 3: 2.
An S.Y., Min S.K., Cha I.H., Choi Y.L., Cho Y.S., Kim C.H. & Lee Y.C. 2002. Decolorization of triphenylmethane and azo dyes by Citrobacter sp. Biotechnol. Lett. 24: 1037–1040
Asgher M. & Bhatti H.N. 2012. Evaluation of thermodynamics and effect of chemical treatments on sorption potential of Citrus waste biomass for removal of anionic dyes from aqueous solutions. Ecol. Eng. 38: 79–85
Balsano C. & Alisi A. 2009. Antioxidant effect of natural bioactive compounds. Curr. Pharm. Design 15: 3063–3073
Bose B., Motiwale L. & Rao K.V.K. 2005. DNA damage and G2/M arrest in Syrian hamster embryo cells during Malachite green exposure are associated with elevated phosphorylation of E.K. and JNK1. Cancer Lett. 230: 260–270
Burhans W.C., Weinberger M., Marchetti M. A., Ramachandran L., D’Urso G. & Huberman J. A. 2003. Apoptosis-like yeast cell death in response to DNA damage and replication defects. Mutat. Res. 532: 227–243
Couto S.R. 2009. Dye removal by immobilised fungi. Biotechnol. Advan. 27: 227–235
Culp S. & Beland F. 1996. Malachite green: a toxicological review. Int. J. Toxicol. 15: 219–238
Deng D., Guo J., Zeng G. & Sun G. 2008. Decolorization of anthraquinone, triphenylmethane and azo dyes by a new isolated Bacillus cereus strain DC11. Int. Biodeterior. Biodegrad. 62: 263–269
Dhamgaye S., Devaux F., Manoharlal R., Vandeputte P., Shah A.H., Singh A., Blugeon C., Sanglard D. & Prasad R. 2012. In vitro effect of malachite green on Candida albicans involves multiple pathways and transcriptional regulators UPC2 and STP2. Antimicrob. Agents Chemother. 56: 495–506
Hauptmann P., Riel C., Kunz-Schughart L.A., Fröhlich K.U., Madeo F. & Lehle L. 2006. Defects in N-glycosylation induce apoptosis in yeast. Mol. Microbiol. 59: 765–778
Jadhav J.P. & Govindwar S.P. 2006. Biotransformation of malachite green by Saccharomyces cerevisiae MTCC 463. Yeast 23: 315–323
Jakubowski W., Biliński T. & Bartosz G. 2000. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic. Biol. Med. 28: 659–664
Jones J.J. & Iii J.O.F. 2003. Decolorization of malachite green and crystal violet by waterborne pathogenic mycobacteria. Antimicrob. Agents Chemother. 47: 2323–2326
Khandare R.V., Kabra A.N., Awate A.V. & Govindwar S.P. 2013. Synergistic degradation of diazo dye Direct Red 5B by Portulaca grandiflora and Pseudomonas putida. Int. J. Environ. Sci. Technol. 10: 1039–1050
Lowry O.H., Rosebrough N.J., Farr A.L. & Randall R.J. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275
Madeo F., Fröhlich E., Ligr M., Grey M., Sigrist S.J., Wolf D.H. & Fröhlich K.U. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145: 757–767
Mahudawala D.M., Redkar A.A., Wagh A., Gladstone B. & Rao K. V. 1999. Malignant transformation of Syrian hamster embryo (SHE) cells in culture by malachite green: an agent of environmental importance. Indian J. Exp. Biol. 37: 904–918
Mukherjee S., Pawar N., Kulkarni O., Nagarkar B., Thopte S., Bhujbal A. & Pawar P. 2011. Evaluation of free-radical quenching properties of standard Ayurvedic formulation Vayasthapana Rasayana. BMC Complement. Altern. Med. 11: 38.
Panandiker A., Fernandes C. & Rao K.V. 1992. The cytotoxic properties of malachite green are associated with the increased demethylase, aryl hydrocarbon hydroxylase and lipid peroxidation in primary cultures of Syrian hamster embryo cells. Cancer Lett. 67: 93–101
Panandiker A., Fernandes C., Rao T.K. & Rao K. V. 1993. Morphological transformation of Syrian hamster embryo cells in primary culture by malachite green correlates well with the evidence for formation of reactive free radicals. Cancer Lett. 74: 31–36
Parekh J. & Chanda S. V. 2007. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 31: 53–58
Perrone G.G., Tan S.X. & Dawes I.W. 2008. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta 1783: 1354–1368
Ren S., Guo J., Zeng G. & Sun G. 2006. Decolorization of triphenylmethane, azo, and anthraquinone dyes by a newly isolated Aeromonas hydrophila strain. Appl. Microbiol. Biotech-nol. 72: 1316–1321
Safarik I., Ptackova L. & Safarikova M. 2002. Adsorption of dyes on magnetically labeled baker’s yeast cells. Eur. Cells Mater. 3: 52–55
Sudova E., Machova J., Svobodova Z. & Vesely T. 2007. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Vet. Med. (Praha) 52: 527–539
Vasdev K., Kuhad R.C. & Saxena R.K. 1995. Decolorization of triphenylmethane dyes by the bird’s nest fungus Cyathus bulleri. Curr. Microbiol. 30: 269–272
Wang J., Qiao M., Wei K., Ding J., Liu Z., Zhang K.Q. & Huang X. 2011. Decolorizing activity of malachite green and its mechanisms involved in dye biodegradation by Achromobacter xylosoxidans MG1. J. Mol. Microbiol. Biotechnol. 20: 220–227
Wu J., Jung B.G., Kim K.S., Lee Y.C. & Sung N.C. 2009. Isolation and characterization of Pseudomonas otitidis WL-13 and its capacity to decolorize triphenylmethane dyes. J. Environ. Sci. 21: 960–964
Acknowledgements
Authors are thankful to DBT, New Delhi, for funding in the form of DBT-SUK IPLS Program through grant No. BT/PR4572/INF/22/147/2012, SAP-DRS II Program, UGC New Delhi, for infrastructure facility and DSTPURSE program (Grant No.: SR/PURSE/2010) funded by DST, New Delhi, and funds for providing fellowship to one of the authors (SPB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biradar, S.P., Rane, N.R., Patil, T.S. et al. Herbal augmentation enhances malachite green biodegradation efficacy of Saccharomyces cerevisiae. Biologia 71, 475–483 (2016). https://doi.org/10.1515/biolog-2016-0069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2016-0069