Abstract
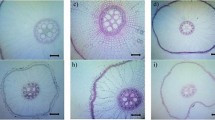
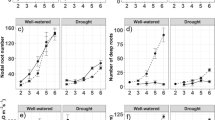
Stipa lagascae R. & Sch. (perennial bunchgrass) is one of the most promising steppic species for arid and desert lands of Tunisia. The present study was designed to study the effect of drought on root and leaf anatomy, water relationship, and the growth of three- month-old S. lagascae plants, submitted to water deficit (5, 10, 15, 20, 30 days of withheld irrigation) and grown in pots in greenhouse conditions. The results show that water deficit treatments reduced the biomass accumulation (MS) and leaf water potential (Ψw) of plants. However, leaf relative water content (RWC) decreased significantly only at severe drought. The root’s anatomical features showed reduced root cross-sectional diameter under water deficit. Conversely, epidermis was unaffected by water stress. Moderate and/or severe water deficit (20–30 days) reduced significantly the cortex thickness, cortical cell size, stele diameter, xylem vessel diameter and the stele/root cross-sectional ratio, while the number of cortical cells increased for severe water deficit. The cuticles and mesophyll of S. lagascae was thickened by moderate to severe drought and the entire lamina thickness was increased significantly by 5.8% only after 30 days of water deficit while epidermis was unaffected by water deficit. However, severe water deficit (30 days) decreased the width and the length of the bundle sheath. At the same time, the mesophyll cells size and both the xylem and phloem vessels diameter diminished by 12, 16.8 and 17.5%, respectively. Leaf rolling occurs as a response to water deficit and its level increases as the drought period is progressing in plants while reduced bulliform cells size occurred only at severe water deficit. Our findings suggest a complex network of root and leaf anatomical adaptations such as a reduced vessel size with lesser cortical and mesophyll parenchyma formation and increased leaf rolling. These proprieties are required for the maintenance of water potential and energy storage under water stress which can improve the resistance of S. lagascae to survive in extremely arid areas.
Similar content being viewed by others
References
Abernethy G.A., Fountain D.W. & Mcmanus M.T. 1998. Observations on the leaf anatomy of Festuca noyaezelandiae and biochemical response to a water deficit. N. Z. J. Bot. 36: 113–123.
Akram M. 2011. Growth and yield components of wheat under water stress of different growth stages. Bangl. J. Agril. Res. 36: 455–468.
Alvarez J.M., Rocha J.F. & Machado S.R. 2008. Bulliform cells in Loudetiopsis chrysothrix (Nees) Conert and Tristachya leiostachya Nees (Poaceae): Structure in relation to function. Braz. Arch. Biol. Technol. 51: 113–119.
Arnold D.H. & Mauseth J.D. 1997. Effetct of environmental factors on developpement of wood. Amer. J. Bot. 86: 367–371.
Athar H. & Ashraf M. 2005. Photosynthesis under drought stress, pp. 795-810. In: Pessarakli M. (ed.), Handbook Photosynthesis, second ed. CRC Press, New York, USA.
Bacelar E.A., Correia C.M., MoutinhoPereira J.M., Goncalves B.C., Lopes J.I. & TorresPereira J.M. 2004. Sclerophylly and leaf anatomical traits of five fieldgrown olive cultivars growing under drought conditions. Tree Physiol. 24: 233–239.
Balsamo R.A., Willigen C.V., Bauer A.M. & Farrant J. 2006. Drought tolerance of selected Eragrostis species correlates with leaf tensile properties. Ann. Bot. 97: 985–991.
Ben Ahmed C., Ben Rouina B. & Boukhris M. 2007. Effects of water deficit on olive trees cv. Chemlali under field conditions in arid region in Tunisia. Sci. Hort. 113: 267–277.
Bohnert H.J., Nelson D.E. & Jensen R.G. 1995. Adaptations to environmental stresses. Plant Cell 7: 1099–1111.
Bongi G. & Loreto F. 1989 Gas exchange properties of saltstressed olive (Olea europaea L.) leaves. Plant Physiol. 90: 1408–1416.
Bosabalidis A.M. & Kofidis G. 2002. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 163: 375–379.
Boughalleb F. & Hajlaoui H. 2011. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zamlati and Chemlali). Acta Physiol. Plant. 33: 53–65.
Burghardt M., Burghardt A., Gall J., Rosenberger C. & Riederer M. 2008. Ecophysiological adaptations of water relations of Teucrium, chamaedrys L. to the hot and dry climate of xeric limestone sites in Franconia (Southern Germany). Fiora 203: 3–13.
Burnett S.E., Thomas P.A. & Van Iersel M.W. 2000. Postegermination with PEG-8000 reduces growth of Salvia and manigolds. Hortscience 40: 675-679
Burnett S.E., Pennisi S.V., Thomas P.A. & van Iersel M.W. 2005. Controlled drought affects morphology and anatomy of Salvia splendens. J. Amer. Soc. Hort. Sci. 130: 775–781.
Bussotti F., Bottacci A., Bartolesi A., Grossoni P. & Tani C. 1995. Morphoanatomical alterations in leaves collected from beech trees (Facus sylvatica L.) in conditions of natural water stress. Environ. Exp. Bot. 35: 201–213.
Chartzoulakis K., Patakas A. & Bosabalidis A. 1999. Changes in water relations, photosynthesis and leaf anatomy induced by intermittent drought in two olive cultivars. Environ. Exp. Bot. 42: 113–120.
Child R.D., Summers J.E., Babij J., Farrent J.W. & Bruce D.M. 2003. Increased resistance to pod chatter is associated with changes in the vascular structure in pods of a resynthesized Brassica napus line. J. Exp. Bot. 54: 1919–1930.
Clifford S.C., Arndt S.K., Popp M. & Jones H.G. 2002. Mucilages and polysaccharides in Ziziphus species (Rhamnaceae): localization, composition and physiological roles during drought stress. J. Exp. Bot. 53: 131–138.
Cutler D.F., Botha T. & Stevenson D.W. 2007. Plant Anatomy. An applied approach. Blackwell Publishing, Australia.
Da Silva S., Castro E.M. & Soares A.M. 2003. Effects of different water regimes on the anatomical characteristics of roots of grasses promising for revegetation of areas surrounding hydroelectric reservoirs. Ciénc Agrotec Lavras 27: 393–397.
Dickison W.C. 2000. Integrative Plant Anatomy. Harcourt Academie Press, San Diego, San Francisco, New York, Boston, London, Toronto, Sydney, Tokyo.
Domingo R., RuizSánchez M.C., SánchezBlanco M.J. & Torrecillas A. 1999. Water relations, growth and yield of Fino lemon trees under regulated deficit irrigation. Irrig. Sci. 16: 115–123.
ElAfry M.M., ElNady M.F. & Abdelmonteleb E.B. 2012. Anatomical studies on droughtstressed wheat plants (TrifÁcum, aestivum L.) treated with some bacterial strains. Acta Biol. Szeg. 56: 165–174.
Esau K. 1977. Anatomy of Seed Plants. 2nd ed. New York, John Wiley and Sons, pp. 351–353.
Farouk S. & Amany A.R. 2012. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egy. J. Biol. 14: 14–26.
Fini A., Guidib L., Ferrini F., Brunettia C., Di Ferdinandoa M., Biricolti S., Pollastri S., Calamaia L. & Tattini M. 2012. Drought stress has contrasting effects on antioxidant enzymes activity and phenyl propanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair. J. Plant Physiol. 169: 929–939.
Galle A., Haldimann P. & Feller U. 2007. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 174: 799–810.
Gindaba J., Rozanov A. & Negash L. 2004. Response of seedlings of two Eucalyptus and three deciduous tree species from Ethiopia to severe water stress. For. Ecol. Manage. 201: 119–129.
Guerfel M., Baccouri O., Boujnah D., Chaibi W. & Zarrouk M. 2009. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 119: 257–263.
Jacobsen A.L., Ewers F.W., Pratt R.B., Paddock W.A. & Davis S.D. 2005. Do xylem fibers affect vessel cavitation resistance. Plant Physiol. 139: 546–556.
James S.A. & Bell D.T. 1995. Morphology and anatomy of leaves of Eucalyptus camaldulensis clones: variation between geographically separated locations. Aust. J. Bot. 43: 415–433.
Kadioglu A. & Terzi R. 2007. A dehydration avoidance mechanism: Leaf rolling. Bot. Rev. 73: 290–302.
Kamel A. & Loser D.M. 1995. Contribution of carbohydrates and other solutes to osmotic adjustment in wheat leaves under water stress. J. Plant Physiol. 145: 363–366.
Kofidis G., Bosabalidis A.M. & Chartzoulakis K. 2004. Leaf anatomical alterations induced by drought stress in two avocado cultivars. J. Biol. Res. 1: 115–120.
Kramer J. & Boyer J.S. 1995. Water Relation of Plants and Soils. Elsevier Science (USA), Acad. Press, San Diego, CA, 495 pp.
Kutlu N., Terzi R., Tekeli C., Senel G., Battal P. & Kadioglu A. 2009. Changes in anatomical structure and levels of endogenous phytohormones during leaf rolling in Ctenanthe setosa. Turk. J. Biol. 33: 115–122.
Lecoeur J. & Sinclair T.R. 1996. Field pea transpiration and leaf growth in response to soil water de ficits. Crop Sci. 36: 331–335.
Le fioch E., Neffati M., Chaieb M., fioret C. & Pontanier R. 1999. Rehabilitation experiment at Menel Habib, Southern Tunisia. Arid Soil Res. Rehab. 13: 357–368.
Lersten N.R. & Curtis J.D. 2001. Idioblasts and other unusual internal foliar secretary structures in Scrophulariaceae. Plant Syst. Evol. 227: 63–73.
Levitt J. 1972. Responses of Plants to Environmental Stresses. Academie Press, New York, pp. 31–47.
Li F.L., Bao W.K. & Wu N. 2011. Morphological, anatomical and physiological responses of Campylotropis polyantha (Francii.) Schindl. seedlings to progressive water stress Sci. Hortic. 127: 436–443.
Liu F. & Stiitzel H. 2004. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to water stress. Sci. Hortic. 102: 15–27.
Lo Gullo M.A., Salleo S., Piaceri E.C. & Rossor. 1995. Relations between vulnerability to xylem embolism and xylem conduit dimensions in young trees of Quercus cerris. Plant Cell Eniviron. 18: 661–669.
Lux A., Luxova M., Abe J. & Morita S. 2004. Root cortex: structural and functional variability and responses to environmental stress. Root Res. 13: 117–131.
Makbul S., Turkmen Z., Coskuncelebi K. & Beyazoglu O. 2008. Anatomical and pollen characters in the genus Epilobium, L. (Onagraceae) from northeast anatolia. Acta Biol. Cracov. Bot. 50: 57–67.
Makbul S., Saruhan G.N., Durmus N. & Guven S. 2011. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Bot. 35: 369–377.
Matsuda K. & Rayan A. 1990. Anatomy: A key factor regulating plant tissue response to water stress. In: Kafternan F. (ed.), Environment Injury to Plants, San Diego: Academie Press, 290 pp.
Medrano H., Escalona J.M., Bota J., Gulias J. & fiexas J. 2002. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal sonductance as a reference parameter. Ann Bot. 89: 895–905.
Moulia B. 1994. Biomechanics of leaf rolling. Biomimetics 2: 267–281.
Nawazish S., Hameed M. & Naurin S. 2006. Leaf anatomical adaptations of Cenchrus ciliaris L. from the salt range, Pakistan against drought stress. Pak J. Bot. 38: 1723–1730.
Nicotra A.B., Babicka N. & Westoby N. 2002. Seedling root anatomy and morphology: an examination of ecological differentiation with rainfall using phylogenetically independent contrasts. Oecologia 130: 136–145.
Niu G., Rodriguez D., Mendoza M., Jifon J. & Ganjegunte G. 2012. Reponses of Jatropha curcas to salt and drought stresses. Inter. J. Agronomy. Academie Editor, 7 pp.
O’Connor T.G. 1991. Local extinction in perennial grasslands: A lifehistory approach. The Amer. Naturalist 137: 753–773.
O’Connor T.G. 1996. Hierarchical control over seedling recruitment of the bunchgrass Themeda triandra in a semiarid savanna. J. App. Ecol. 33: 1094–1106.
Ogbonnaya C.I., Nwalozie MC.., RoyMacauley H. & Annerose D. J.M. 1998. Growth and water relations of Kenaf (Hibiscus cannabinus L.) under water deficit on a sandy soil. Ind. Crops Prod. 8: 65–76.
Olmos E., SanchezBlanco M.J., Fernandez T. & Alarcon J.J. 2007. Subcellular effects of drought stress in Rosmarinus officinalis. Plant Biol. 9: 77–84.
PeńaValdivia C.B., SánchezUrdaneta A.B., Trejo C., Aguirre R.J.R. & Cardenas E. 2005. Root anatomy of drought sensitive and tolerant maize (Zea mays L.) seedlings under different water potentials. Cereal Res. Comm. 33: 705–712.
PeńaValdivia C.B. & SánchezUrdaneta A.B. 2009. Effects of substrate water potential in root growth of Agáve salmiana Otto ex SalmDyck seedlings. Biol. Res. 42: 239–248.
PeńaValdivia C.B., SánchezUrdaneta A.B., Rangel J.M., Muńoz J.J., GarcíaNava R. & Velázquez R.C. 2010. Anatomical root variations in response to water deficit: wild and domesticated common bean (Phaseolus vulgaris L.) Biol. Res. 43: 417–427.
Price A.H., Young E.M. & Tomos A.D. 1997. Quantitative trait loci associated with stomatal conductance, leaf rolling and heading date mapped in upland rice (Oryza sativa). New Phytol. 137: 83–91.
Reddy A.R., Chiatanya K.V. & Vivekanandan M. 2004. Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 161: 1189–1202.
Rosales M., CuellarOrtiz S., AcostaGallegos J. & Cavarrabias A. 2012. Physiological traits related to terminal drought resistance in common bean Phaseolus vulgaris L. J. Sci. Food Agric. 93: 324–331.
Sairam R.K. & Tyagi A. 2004. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 6: 407–421.
Saleem M., Lamkemeyer T., Schutzenmeister M.T., Sakai H., Piepho H.P., Nordheim A. & Hochholdinge F. 2010. Speci fication of cortical parenchyma and stele of maize primary roots by asymmetric levels of auxin, cytokinin, and cytokininregulated proteins. Plant Physiol. 152: 4–18.
Salisbury F.B. & Ross C.W. 1992. Plant Physiology. Wadsworth Publishing Company, Belmont.
Sam O., Jeréz E. & Varela M. 1996. Caracteristicas anatomicas de hojas de apa (Solanum, tuberosum L.) y tomate (Lycopersycon esculentum Mill.) can diferentes grados de tolerancia a estres de humedad y temperatura. Cultivos Tropicales 17: 32–38.
Sam O., Jeréz E., Dell’Amico J. & RuizSánchez M.C. 2000. Water stress induced changes in anatomy of tomato leaf epidermis. Biol. Plant. 43: 275–277.
Scholz H. 1991. Stipa tunetana, eine neue Artaus Tunesien, und das St. lagascae Aggregat (Gramineae). Willdenowia 26: 225–228.
Schultz H.R. & Matthews M.A. 1988. Resistance to water transport in shoots of VifÁs vinifero, L. Plant Physiol. 88: 718–724.
Selim H. & ElNady M. 2011. Physioanatomical responses of drought stressed tomato plants to magnetic field. Acta Astro. 2: 1–9.
Shao H.B., Chu L.Y., Jaleel CA. & Zhao C.X. 2008. Water deficit stress induced anatomical changes in higher plants. C. R. Biol. 331: 215–225.
Shilei G., Sheng Z. & Hong W. 2002. Anatomical characters of stems and leaves of three lawn grasses. J. Trop. Subtrop. Bot. 10: 145–151.
Sibounheuang V., Basnayake J. & Fukai S. 2006. Genotypic consistency in the expression of leaf water potential in rice (Oryzo, sativa L.). Field Crops Res. 97: 142–154.
Silva S., Soares A.M., Oliveira L.E.M. & Magalháes P.C. 2001. Respostas fisiológicas de gramíneas promissoras para revegetação ciliar de reservatórios hidrelétricos, submetidas à deficięncia hídrica. Cięncia Agrotécnica 25: 124–133.
Singh A., Shamim M. & Singh K.N. 2013. Genotypic variation in root anatomy, starch accumulation, and protein induction in upland rice (Oryzo, sativa) varieties under water stress. Agric. Res. 2: 24–30.
Srivastava L.M. 2001. Plant growth and development. Digital stock Inc., 718 pp.
Stiller V., Lafitte H.R. & Sperry J.S. 2003. Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiol. 132: 1698–1706.
Stolf R., Medri M.E., Pimenta J.A., Boeger M.R.T., Dias J., Lemos N.G., de Oliveira M.C.N., Brogin R.L., Yamanaka N., Neumaier N., Farias J.R.B. & Nepomuceno A.L. 2009. Morphoanatomical and micromorphometrical evaluations in soybean genotypes during water stress. Braz. Arch Biol. Technol. 52: 1313–1331.
Syvertsen J.F., Lloyd J., McConchie C., Kriedemann P.E. & Farquhar G.D. 1995. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell Environ. 18: 149–157.
Twumasi P., van Ieperen W., Woltering E.J., Emons A.M.C., Schel J.H.N., Schel J.F.H., van Meeteren U. & van Marwijk D. 2005. Effects of water stress during growth on xylem anatomy, xylem functioning and vaše life in three Zinnia elegans cultivars. Acta Hort. 669: 303–311.
Uga Y., Okuno K. & Yano M. 2008. QTLs underlying natural variation in stele and xylem structures of rice root. Breeding Sci. 58: 7–14.
van Ieperen W., Nijsse J., Keijzer C.J. & Van Meeteren U. 2001. Induction of air embolism in xylem conduits of prede fined diameter. J. Exp. Bot. 52: 981–991.
Vasellati V., Oesterheld M., Medan D. & Loreti J. 2001. Effects of flooding and drought on the anatomy of Paspalum, dilatatum. Ann. Bot. 88: 355–360.
Wang W., Vincour B. & Altman A. 2003. Plant responses to drought, salinity and extréme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1–14.
Xiang J.J., Zhang G.H., Qian Q. & HongWei X.H.W. 2012. Encodes a putative glycosylphosphatidylinositolanchored protein and modulates rice leaf rolling by regulating the formation of bulliform cells. Plant Physiol. 159: 1488–1500.
Zhu J.K. 2001. Plant salt tolerance. Trends Plant Sci. 6: 66–71.
Zimmermann M.H. 1983. Xylem Structure and the Ascent of Sap. SpringerVerlag, Berlin, Heidelberg, New York, Tokyo, 143 pp.
Acknowledgements
We gratefully acknowledge all the technical staff of the Arid Regions Institute-Medenine (IRA) for their help to conductingthese experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boughalleb, F., Abdellaoui, R., Hadded, Z. et al. Anatomical adaptations of the desert species Stipa lagascae against drought stress. Biologia 70, 1042–1052 (2015). https://doi.org/10.1515/biolog-2015-0125
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2015-0125