Abstract
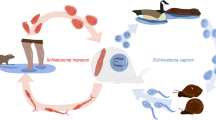
Auto-infection is a life history strategy used by many parasitic organisms, including digenetic trematodes. The process of autoinfection most frequently involves the transfer of a life cycle stage of the parasite from one site to another inside the same host, usually accompanied by morphological transformation. Moreover, among trematodes, the stage being transferred may also move from one host to another in completing the life cycle, i.e., an indirect cycle. Echinostoma spp. parasites offer the opportunity to study auto-infection because they utilize gastropods as both first and second intermediate hosts. Rejection of a null model predicting independent infection of first and second intermediate larval stages coupled with the presence of rediae being the best predictor of metacercariae prevalence and intensity suggests that auto-infection by Echinostoma spp. cercariae is occurring in their molluscan hosts. Shell length was also found to be a significant predictor of metacercariae intensity in the snails hosts, but this is most likely attributed to larger snails being more commonly infected with Echinostoma spp. rediae as opposed to an increased likelihood of cercariae infection. Auto-infection as a life history strategy increases transmission success of the parasite, but may also have negative consequences for the parasite that necessitate auto-infection coupled with the release of cercariae to maximize transmission success and host survival.
Similar content being viewed by others
References
Bolek M.G., Janovy Jr. J. 2008. Alternative life cycle strategies of Megalodiscus temperatus in tadpoles and metamorphosed anurans. Parasite, 15, 396–401. DOI: 10.1051/parasite/2008153396
Bush A.O., Lafferty K.D., Lotz J.M., Schostak A.W. 1997. Para-sitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology, 83, 573–583. DOI: 10.2307/3284227
Chernin E. 1962. The unusual life-history of Daubaylia potomaca (Nematoda: Cephalobidae) in Australorbis glabratus and in certain other freshwater snails. Journal of Parasitology, 52, 459–481. DOI: 10.1017/S0031182000027268
Detwiler J.T. 2010. The molecular ecology of echinostome trematodes: Elucidating the phylogenetics and transmission dynamics of a freshwater helminth parasite. Ph.D. Dissertation. Purdue University, West Lafayette, Indiana, U.S.A
Detwiler J.T., Minchella D.J. 2009. Intermediate host availability masks the strength of experimentally derived colonization patterns in echinostome trematodes. International Journal for Parasitology, 39, 585–590. DOI: 10.1016/j.ijpara.2008.10.008
Dybdahl M.F., Lively C.M. 1998. Host-parasite coevolution: Evidence for rare advantage and time-lagged selection in a natural population. Evolution, 52, 1057–1066. DOI: 10.2307/2411236
Esch G.W., Barger M.A., Fellis K.J. 2002. The transmission of digenetic trematodes: Style, elegance, complexity. Integrative and Comparative Biology, 42, 304–312. DOI: 10.1093/icb/42.2.304
Esch G.W., Fernandez J.C. 1994. Snail-trematode interactions and parasite community dynamics in aquatic systems: A review. American Midland Naturalist, 131, 209–237.
Esteban J.G., Muñoz-Antoli C. 2009. Echinostomes: Systematics and life cycles. In: (Eds. B.R. Fried and R. Toledo) The biology of echinostomes: From the molecule to the community. Springer LLC, New York, New York, 1–34
Evans N.A., Gordon D.M. 1983. Experimental observations on the specificity of Echinoparyphium recurvatum toward second intermediate hosts. Parasitology Research, 69, 217–222. DOI: 10.1007/BF00926956
Fried B., Bennett M.C. 1979. Studies on encystment of Echinostoma revolutum cercariae. Journal of Parasitology, 65, 38–40. DOI: 10.2307/3280199
Garcia H.H., Gonzalez A.E., Evans C.A.W., Gilman R.H. 2003. Taenia solium cysticercosis. The Lancet, 361, 547–556. DOI: 10.1016/S0140-6736(03)14117-7
Haas W., Haberl B. 1997. Host recognition by trematode miracidia and cercaria. In: (Eds. B. Fried and T.K. Graczyk) Advances in trematode biology. CRC Press, Boca Raton, Florida, 197–227
Haas W., Haberl B., Kalbe M., Körner M. 1995. Snailhost-finding by miracidia and cercariae: Chemical host cues. Parasitology Today, 11, 468–472. DOI: 10.1016/0169-4758(95)80066-2
Haberl B., Körner M., Spengler Y., Hertel J., Kalbe M., Haas W. 2000. Host-finding in Echinostoma caproni: Miracidia and cercariae use different signals to identify the same snail species. Parasitology, 120, 479–486. DOI: 10.1017/S0031182099005697
Johnson P.T.J., McKenzie V.J. 2009. Effects of environmental change on helminth infections in amphibians: Exploring the emergence of Ribeiroia and Echinostoma infections in North America. In: (Eds. B.R. Fried and R. Toledo) The biology of echinostomes: From the molecule to the community. Springer LLC, New York, New York, 249–280
Jokela J., Lively C.M. 1995. Spatial variation in infection by digenetic trematodes in a population of freshwater snails (Potamopyrgus antipodarum). Oecologia, 103, 509–517. DOI: 10.1007/BF00328690
Kaneko J.J., Yamada R., Brock J.A., Nakamura R.M. 2006. Infection of tilapia, Oreochromis mossambicus (Trewavas), by a marine monogenean, Neobenedenia melleni (MacCallum, 1927) Yamaguti, 1963 in Kaneohe Bay, Hawaii, USA, and its treatment. Journal of Fish Disease, 11, 295–300. DOI: 10.1111/j.1365-2761.1988.tb01225.x
Kuris A.M., Warren J. 1980. Echinostome cercarial penetration and metacercarial encystment as mortality factors for a second intermediate host, Biomphalaria glabrata. Journal of Parasitology, 66, 630–635. DOI: 10.2307/3280520
Kuris A.M., Lafferty K.D. 1994. Community structure: Larval trematodes in snail hosts. Annual Review of Ecology, Evolution, and Systematics, 25, 189–217. DOI: 10.1146/annurev.es.25.110 194.001201
Lo C.T. 1975. Echinostoma macrorchis: Life history, population dynamics of intramolluscan stages, and the first and second intermediate hosts. Journal of Parasitology, 81, 569–576. DOI: 10.2307/3283855
Lo C.T., Cross J.H. 1975. Observations on the host-parasite relations between Echinostoma revolutum and lymnaeid snails. Chinese Journal of Microbiology and Immunology, 8, 241–252. DOI: 1243895
MacKenzie K. 1981. The effect of Eimeria sp. infection on the condition of blue whiting, Micromesistius poutassou (Risso). Journal of Fish Disease, 4, 473–486. DOI: 10.1111/j.1365-2761.1981.tb01160.x
McCarthy A.M., Kanev I. 1990. Pseudechinoparyphium echinatum (Digenea: Echinostomatidae): Experimental observations on cercarial specificity toward second intermediate hosts. Para-sitology, 100, 423–428. DOI: 10.1017/S0031182000078719
McCoy K.D., Buolinier T., Tirard C., Michalakis Y. 2003. Host-dependent genetic structure of parasite populations: Differential dispersal of seabird tick host races. Evolution, 57, 288–296. DOI: 10.1554/0014-3820(2003)057[0288:HDGSOP]2.0.CO;2
Morley N.J., Crane M., Lewis J.W. 2004. Influence of cadmium exposure on the incidence of first intermediate host encyst-ment by Echinoparyphium recurvatum cercariae in Lymnaea peregra. Journal of Helminthology, 78, 329–332. DOI: 10.1079/JOH2004267
Neva F.A. 1986. Biology and immunology of human strongyloidiasis. Journal of Infectious Disease, 153, 397–406. DOI: 10.1093/infdis/153.3.397
Olsen O.W. 1937. Description and life history of the trematode Haplotrana utahensis sp. nov. (Plagiochiidae) from Rana pretiosa. Journal of Parasitology, 23, 13–28
Pechenik J.A., Fried B. 1995. Effect of temperature on survival and infectivity of Echinostoma trivolvis cercariae: A test of the energy limitation hypothesis. Parasitology, 111, 373–378. DOI: 10.1017/S0031182000081920
Poulin R., Cribb T.H. 2002. Trematode life cycles: Short is sweet? Trends in Parasitology, 18, 176–183. DOI: 10.1016/S1471-4922(02)02262-6
Rankin Jr. J.S. 1944. A review of the trematode genus Glypthelmins Stafford 1905, with an account of the life cycle of G. quieta (Stafford, 1900) Stafford, 1905. Transactions of the American Microscopical Society, 63, 30–43
Rees F.G. 1932. An investigation into the occurrence, structure and life histories of the trematode parasites of four species of Lymnaea (Lymnaea truncatula (Mull), Lymnaea palustris (Mull), and Lymnaea stagnalis (Linne)), and Hydrobia jenkinsi (Smith) in Glamorgan and Monmouth. Proceedings of the Zoological Society of London, 1932, 1–32
Sandland G.J., Goater C.P., Danylchuk A.J. 2001. Population dynamics of Ornithodiplostomum ptychocheilus metacercariae in fathead minnows (Pimephales promelas) from four northern Alberta lakes. Journal of Parasitology, 87, 744–748. DOI: 10.1645/0022-3395(2001)087[0744:PDOOPM]2.0.CO;2
Sorensen R.E., Minchella D.J. 2001. Snail-trematode life history interactions: Past trends and future directions. Parasitology, 123, S3–S18. DOI: 10.1017S0031182001007843.
Sousa W.P. 1983. Host life history and the effect of parasitic castration on growth: A field study of Cerithidea californica Haldeman (Gastropoda: Prosobranchia) and its trematode parasites. Journal of Experimental Marine Biology and Ecology, 73, 273–296. DOI: 10.1016/0022-0981(83)90051-5
Sullivan J.J., Byrd E.E. 1970. Choledocystus pennsylvaniensis: Life history. Transactions of the American Microscopical Society, 89, 384–396
Trouve S., Renaud F., Durand P., Jourdane J. 1996. Selfing and out-crossing in a parasitic hermaphrodite helminth (Trematoda, Echinostomatidae). Heredity, 77, 1–8. DOI: 10.1038/hdy. 1996.101
Tzipori S. 1988. Cryptosporidiosis in perspective. Advances in Par-asitology, 27, 63–129
Vareille-Morel C., Esclaire F., Hourdin P., Rondelaude D. 1993. Internal metacercarial cysts of Fasciola hepatica in the pulmonate snail Lymnaea truncatula. Parasitology Research, 79, 259–260
Wesenberg-Lund, C. 1934. Contributions to the development of the Trematoda. Part 2. The biology of the freshwater cercariae in Danish freshwaters. Memoires de l’Academie Royale des Sciences et des Lettres de Danemark, Copenhague, Section des Sciences, 9me Serie, Tome 5, 1–223
Whittington I.D. 1997. Reproduction and host-location among the parasitic Platyhelminthes. International Journal for Parasitology, 27, 705–714
Zimmermann M.R., Luth K.E., Camp L.E., Esch G.W. 2011a. Population and infection dynamics of Daubaylia potomaca (Nematoda: Rhabditida) in Helisoma anceps. Journal of Parasitology, 97, 384–388. DOI: 10.1645/GE-2603.1
Zimmermann M.R., Luth K.E., Esch G.W. 2011b. The unusual life cycle of Daubaylia potomaca, a nematode parasite of Helisoma anceps. Journal of Parasitology, 97, 430–434. DOI: 10.1645/GE-2604.1
Zimmermann M.R., Luth K.E., Esch G.W. 2011c. Complex interactions among a nematode parasite (Daubaylia potomaca), a commensalistic annelid (Chaetogaster limnaei limnaei), and trematode parasites in a snail host (Helisoma anceps). Journal of Parasitology, 97, 788–791. DOI: 10.1645/GE-2733.1
Zimmermann M.R., Luth K.E., Esch G.W. 2014. Differences in snail ecology lead to infection pattern variation of Echinostoma spp. larval stages. Acta Parasitologica, 59, 502–509. DOI: 10.2478/s11686-014-0275-6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zimmermann, M.R., Luth, K.E. & Esch, G.W. Auto-infection by Echinostoma spp. cercariae in Helisoma anceps. Acta Parasit. 60, 700–706 (2015). https://doi.org/10.1515/ap-2015-0099
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/ap-2015-0099