Abstract
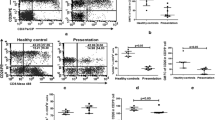
Adenosine deaminase (ADA) which degrades adenosine to inosine, is known to be pro-inflammatory molecule in many diseases. Adenosine suppresses the functioning of the immune system and thus promotes dissemination of the parasite. In our previous finding, the level of soluble ADA in serum of visceral leishmaniasis (VL) was found to be increased as compared to healthy controls. However, it cannot be fairly interpreted unless their level is demonstrated at the disease site, where the parasite resides. We designed this study to correlate the level of soluble ADA (sADA) with parasitic load at the disease site i.e. bone marrow (BM). We found increased levels of sADA in BM as compared to the unaffected BM. Furthermore, a significant inverse correlation is observed between the parasite load and level of sADA at the disease site.
Similar content being viewed by others
References
Awasthi A., Mathur R.K., Saha B. 2004. Immune response to Leishmania infection. Indian Journal of Medical Research, 119, 238–58
Baba K., Hoosen A.A., Langeland N., Dyrhol-Riise A.M. 2008. Adenosine deaminase activity is a sensitive marker for the diagnosis of tuberculous pleuritis in patients with very low CD4 counts. PLoS One, 3, e2788. DOI: 10.1371/journal.pone.0002788
Baral N., Mehta K.D., Chandra L., Lamsal M., Rijal S., Koirala S. 2005. Adenosine deaminase activity in sera of patients with visceral leishmaniasis in Nepal. Tropical Doctor, 35, 86–8
Burgess L.J., Maritz F.J., Le Roux I., Taljaard J.J. 1996. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest, 109, 414–9. DOI: 10.1378/chest.109.2.414
Ciruela F., Saura C., Canela E.I., Mallol J., Lluis C., Franco R. 1996. Adenosine deaminase affects ligand-induced signalling by interacting with cell surface adenosine receptors. FEBS Letters, 380, 219–23. DOI: 10.1016/0014-5793(96)00023-3
de Almeida Marques-da-Silva E., de Oliveira J.C., Figueiredo A.B., de Souza Lima Junior D., Carneiro C.M., Rangel Fietto J.L., Crocco Afonso L.C. 2008. Extracellular nucleotide metabolism in Leishmania: influence of adenosine in the establishment of infection. Microbes and Infection, 10, 850–7. DOI: 10.1016/j.micinf.2008.04.016
Eltzschig H.K., Weissmuller T., Mager A., Eckle T. 2006. Nucleotide metabolism and cell-cell interactions. Methods in Molecular Biology, 341, 73–87. DOI: 10.1385/1-59745-113-4:73
Gakis C., Calia G., Naitana A., Pirino D., Serru G. 1989. Serum adenosine deaminase activity in HIV positive subjects. A hypothesis on the significance of ADA2. Panminerva medica, 31, 107–13
Giusti G., Gakis C. 1971. Temperature conversion factors, activation energy, relative substrate specificity and optimum pH of adenosine deaminase from human serum and tissues. Enzyme, 12, 417–25
Hall J.G. 1963. Adenosine deaminase activity in lymphoid cells during antibody production. Australian Journal of Experimental Biology and Medical Science, 41, 93–97. DOI: 10.1038/icb.1963.8
Harris S.G., Padilla J., Koumas L., Ray D., Phipps R.P. 2002. Prostaglandins as modulators of immunity. Trends in Immunology, 23, 144–50. DOI: 10.1016/S1471-4906(01)02154-8
Hirschhorn R., Ratech H. 1980. Isozymes of adenosine deaminase. Isozymes Current Topics in Biological Medical Research, 4, 131–57
Olah M.E., Stiles G.L. 1995. Adenosine receptor subtypes: characterization and therapeutic regulation. Annual Review of Pharmacology and Toxicology, 35, 581–606. DOI: 10.1146/annurev.pa.35.040195.003053
Pinheiro C.M., Martins-Duarte E.S., Ferraro R.B., Fonseca de Souza A.L., Gomes M.T., Lopes A.H., Vannier-Santos M.A., Santos A.L., Meyer-Fernandes J.R. 2006. Leishmania amazonensis: Biological and biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Experimental Parasitology, 114, 16–25. DOI: 10.1016/j.exppara.2006.02.007
Rai A.K., Thakur C.P., Singh A., Seth T., Srivastava S.K., Singh P., Mitra D.K.. 2012. Regulatory T cells suppress T cell activation at the pathologic site of human visceral leishmaniasis. PLoS One, 7, e31551. DOI: 10.1371/journal.pone.0031551
Rai A.K., Thakur C.P., Velpandian T., Sharma S.K., Ghosh B., Mitra D.K. 2011. High concentration of adenosine in human visceral leishmaniasis despite increased ADA and decreased CD73. Parasite Immunology, 33, 632–6. DOI: 10.1111/j.1365-3024.2011.01315.x
Roberts M.T. 2005. Current understandings on the immunology of leishmaniasis and recent developments in prevention and treatment. British Medical Bulletin, 115, 75–76. DOI: 10.1093/bmb/ldl003
Rocca B., FitzGerald G.A. 2002. Cyclooxygenases and prostaglandins: shaping up the immune response. International immunopharmacology, 2, 603–30. DOI: 10.1016/S1567-5769(01)00204-1
Sen S., Roy K., Mukherjee S., Mukhopadhyay R., Roy S. 2011. Restoration of IFNγR subunit assembly, IFNγ signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane cholesterol. PLoS Pathogens, 7, e1002229. DOI: 10.1371/journal.ppat.100222
Sypek J.P., Chung C.L., Mayor S.E., Subramanyam J.M., Goldman S.J., Sieburth D.S., Wolf S.F., Schaub R.G. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. The Journal of Experimental Medicine, 177, 1797–802. DOI: 10.1084/jem.177.6.1797
Tripathi K., Kumar R., Bharti K., Kumar P., Shrivastav R., Sundar S., Pai K. 2008. Adenosine deaminase activity in sera of patients with visceral leishmaniasis in India. Clinica Chimica Acta, International Journal of Clinical Chemistry, 388, 135–8. DOI: 10.1016/j.cca.2007.10.022
Ungerer J.P., Oosthuizen H.M., Bissbort S.H., Vermaak W.J. 1992. Serum adenosine deaminase: isoenzymes and diagnostic application. Clinical Chemistry, 38, 1322–6
Wilson D.K., Rudolph F.B., Quiocho F.A. 1991. Atomic structure of adenosine deaminase complexed with a transition-state analog: understanding catalysis and immunodeficiency mutations. Science, 252, 1278–84. DOI: 10.1126/science.1925539
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rai, A.K., Kumar, P., Saini, S. et al. Increased level of soluble adenosine deaminase in bone marrow of visceral leishmaniasis patients: an inverse relation with parasite load. Acta Parasit. 61, 645–649 (2016). https://doi.org/10.1515/ap-2016-0087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/ap-2016-0087