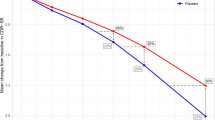
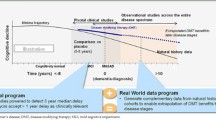
Abstract
Background
Progression in Alzheimer’s disease manifests as changes in multiple biomarker, cognitive, and functional endpoints. Disease progression modeling can be used to integrate these multiple measures into a synthesized metric of where a patient lies within the disease spectrum, allowing for a more dynamic measure over the range of the disease.
Objectives
This study aimed to combine modeling techniques from psychometric research (e.g., item response theory) and pharmacometrics (e.g., hierarchical models) to describe the multivariate longitudinal disease progression for patients with mild-to-moderate Alzheimer’s disease. Additionally, we aimed to extend the subsequent model to make it suitable for clinical trial simulation, with the inclusion of covariates, to explain variability in latent progression (i.e., disease progression) and to aid in the assessment of enrichment strategies.
Design
Multiple longitudinal endpoints in the Alzheimer’s Disease Neuroimaging Initiative database were modeled. This model was validated internally using visual predictive checks, and externally by comparing data from the placebo arms of two Phase 2 crenezumab studies, ABBY (NCT01343966) and BLAZE (NCT01397578).
Setting
The Alzheimer’s Disease Neuroimaging Initiative began in 2004: the initial 5-year study (ADNI-1) was extended by 2 years in 2009 by a Grand Opportunities grant (ADNI-GO), and in 2011 and 2016 by further competitive renewals of the ADNI-1 grant (ADNI-2 and ADNI-3, respectively). This work studies natural progression data from patients with confirmed Alzheimer’s disease. The Phase 2 ABBY and BLAZE trials evaluated the safety and efficacy of crenezumab in patients with mild-to-moderate Alzheimer’s disease.
Participants
From the Alzheimer’s Disease Neuroimaging Initiative database, 305 subjects who had a baseline diagnosis of mild-to-moderate Alzheimer’s disease were included in modeling. From the ABBY and BLAZE studies, 158 patients were included from the studies’ placebo arms.
Measurements
Longitudinal cognitive and functional assessments modeled included the Clinical Dementia Rating (both as Sum of Boxes and individual item scores), the Mini-Mental State Examination, the Alzheimer’s Disease Assessment Scale — Cognitive Subscale, the Functional Activities Questionnaire, the Montreal Cognitive Assessment, and the Rey Auditory Verbal Learning Test. Also included were the imaging variable fluorodeoxyglucose-positron emission tomography and the following magnetic resonance imaging volumetrics: entorhinal, fusiform, hippocampal, intra-cranial, mid-temporal, ventricular, and whole brain.
Results
Applying item response theory approaches in this longitudinal setting showed clinical assessments informing a common disease scale in the following order (from early disease to late disease): Rey Auditory Verbal Learning Test, Functional Activities Questionnaire, Montreal Cognitive Assessment, Alzheimer’s Disease Assessment Scale — Cognitive Subscale 12, Clinical Dementia Rating — Sum of Boxes, and Mini-Mental State Examination. The Clinical Dementia Rating communication and home-and-hobbies items were most informative at earlier disease stages, while memory, orientation, and personal care informed the disease status at later stages. A clinical trial simulation model was developed and accurately described within-sample longitudinal distribution of endpoints. Simplifying the model to use only baseline age, MMSE, and APOEε4 status as predictors, out-of-sample mean progression of ADAS-Cog and CDR Sum of Boxes in the ABBY and BLAZE placebo arms was accurately described; however, the variability in these endpoints was underpredicted and suggests possibility for further model refinement when extrapolating from the ADNI sample to trial data. Clinical trial simulations were performed to exemplify use of the model to investigate hypothetical disease modification effects on the multivariate, longitudinal progression on the Alzheimer’s Disease Assessment Scale — Cognitive Subscale and the Clinical Dementia Rating — Sum of Boxes.
Conclusions
The latent variable structure of item response theory can be extended to capture a variety of scales that are common assessments and indicators of disease status in mild-to-moderate Alzheimer’s disease. These models are not intended to support causal inferences, but they do successfully characterize the observed correlation between endpoints over time and result in concise numerical indices of disease status that reflect the totality of evidence from considering the endpoints jointly. As such, the models have utility for a variety of tasks in clinical trial design, including simulation of hypothetical drug effects, interpolation of missing data, and assessment of in-sample information.





Similar content being viewed by others
Data availability statement: For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Requests for the modeling data underlying this publication requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and company subject matter experts. Direct such requests to global.patient_level_data_sharing@roche.com for consideration. Anonymized records for individual patients across more than one data source external to Roche can not, and should not, be linked due to a potential increase in risk of patient re-identification. Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete list of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
References
Sun B-L, Li W-W, Zhu C, et al. Clinical research on Alzheimer’s disease: progress and perspectives. Neurosci Bull 2018;34:1111–1118.
Hamasaki T, Evans SR, Asakura K. Design, data monitoring, and analysis of clinical trials with co-primary endpoints: a review. J Biopharm Stat 2018;28:28–51.
Samtani M. Disease progression analysis for ADNI MCI subjects utilizing ADAS-cog/11 scores. Creating Consensus Science: New Tools and Tactics for Next-Gen Drug Development. Session IV. Critical Path Institute. 2011. p31-41: https://c-path.org//wp-content/uploads/2013/09/consensussciencequantitativediseaseprogressionmodelsastools.pdf. Accessed 15 July 2022.
Rogers JA, Polhamus D, Gillespie WR, et al. Combining patient-level and summary-level data for Alzheimer’s disease modeling and simulation: a β regression meta-analysis. J Pharmacokinet Pharmacodyn 2012;39:479–498.
Ito K, Ahadieh S, Corrigan B, et al. Disease progression meta-analysis model in Alzheimer’s disease. Alzheimers Dement 2010;6:39–53.
Delor I, Charoin JE, Gieschke R, Retout S, Jacqmin P. Modeling Alzheimer’s disease progression using disease onset time and disease trajectory concepts applied to CDR-SOB scores from ADNI. CPT Pharmacometrics Syst Pharmacol 2013;2:e78.
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414.
O’Bryant SE, Lacritz LH, Hall J, et al. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer’s coordinating center database. Arch Neurol 2010;67:746–749.
Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329.
Li D, Iddi S, Thompson WK, et al. Bayesian latent time joint mixed-effects model of progression in the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement (Amst) 2018;10:657–668.
Ueckert S, Plan EL, Ito K, et al. Improved utilization of ADAS-cog assessment data through item response theory based pharmacometric modeling. Pharm Res 2014;31:2152–2165.
Miller TM, Balsis S, Lowe DA, Benge JF, Doody RS. Item response theory reveals variability of functional impairment within clinical dementia rating scale stages. Dement Geriatr Cogn Disord 2011;32:362–366.
Lowe DA, Balsis S, Miller TM, Benge JF, Doody RS. Greater precision when measuring dementia severity: establishing item parameters for the Clinical Dementia Rating Scale. Dement Geriatr Cogn Disord 2012;34:128–134.
Rencher AC, Christensen WF. Methods of multivariate analysis. 2012. Wiley, Hoboken, New Jersey.
Vandemeulebroecke M, Bornkamp B, Krahnke T, et al. A longitudinal item response theory model to characterize cognition over time in elderly subjects. CPT Pharmacometrics Syst Pharmacol 2017;6:635–641.
Leoutsakos JM, Gross AL, Jones RN, Albert MS, Breitner JCS. ‘Alzheimer’s Progression Score’: development of a biomarker summary outcome for AD prevention trials. J Prev Alzheimers Dis 2016;3:229–235.
Roy J, Lin X. Latent variable models for longitudinal data with multiple continuous outcomes. Biometrics 2000;56:1047–1054.
Tsiatis A, Davidian M. Joint modeling of longitudinal and time-to-event data: an overview. Stat Sin 2004;14:809–834.
Proust-Lima C, Amieva H, Jacqmin-Gadda H. Analysis of Multivariate Mixed Longitudinal Data: A Flexible Latent Process Approach. Br J Math Stat Psychol 2013;66:470–487.
Alzheimer’s Disease Neuroimaging Initiative (ADNI). ADNI Data Package for R. ADNIMERGE 0.0.1. 2021. https://adni.bitbucket.io. Accessed 15 July 2022.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R-project.org/. Accessed 15 July 2022.
Polhamus DG, Rogers JA, Gillespie WR, French J. Clinical dementia rating modeling and simulation: joint progression of CDR and biomarkers in the ADNI cohort. 2013. https://www.metrumrg.com/wp-content/uploads/2017/10/Polhamus_AAIC_2013.pdf. Accessed 15 July 2022.
van der Linden WJ, Pashley PJ. Elements of Adaptive Testing. In: van der Linden WJ, Glas CAW (eds) Item Selection and Ability Estimation in Adaptive Testing. 2009. Springer New York, New York, NY, pp 3–30.
Gastonguay MR, French JL, Heitjan DF, et al. Missing data in model-based pharmacometric applications: points to consider. J Clin Pharmacol 2010;20:63S–74S.
Proust-Lima C, Dartigues J-F, Jacqmin-Gadda H. Joint modeling of repeated multivariate cognitive measures and competing risks of dementia and death: a latent process and latent class approach. Stat Med 2016;35:382–398.
Sevcikova H, Rossini T, L’Ecuyer P. Package ‘rlecuyer’. 2019. https://cran.r-project.org/web/packages/rlecuyer/rlecuyer.pdf. Accessed 15 July 2022.
Acknowledgments
We would like to thank all Alzheimer’s Disease Neuroimaging Initiative (ADNI), ABBY, and BLAZE participants, clinicians, and administrators. Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and the DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation; Araclon Biotech; Bioclinica, Inc.; Biogen; Bristol Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai, Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer, Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research provides funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (https://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of Southern California. Crenezumab was discovered and developed in collaboration with AC Immune SA, Lausanne, Switzerland. Editorial support in the development of this manuscript was provided by Chris Ackroyd, MSc, of Health Interactions, funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Funding
Funding: Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and the DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The Metrum Research Group (Tariffville, CT, USA) and Genentech, Inc. (South San Francisco, CA, USA) were involved in the funding of the work, and the analysis and interpretation of the data. The ABBY and BLAZE studies were funded by Genentech, Inc. Editorial support in the preparation of this manuscript was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest: DGP and JAR are employees of the Metrum Research group. MJD was an employee of Genentech, Inc., a member of the Roche Group, at the time of this work; he is now an employee of Roche Products Pty Limited, Sydney, Australia, and owns stock or stock options in F. Hoffmann-La Roche Ltd. LAH and JYJ are employees of Genentech and own stock in F. Hoffmann-La Roche Ltd. AQ was an employee of Genentech, Inc. and owned stock in Roche Holding Ltd at the time of this study; she is now an employee of AstraZeneca, Gothenburg, Sweden.
Ethical standards: All human procedures were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Additional information
MJD was an employee of Genentech, Inc. at the time of this study
AQ was an employee of Genentech, Inc. at the time of this study
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (https://adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete list of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Polhamus, D.G., Dolton, M.J., Rogers, J.A. et al. Longitudinal Exposure—Response Modeling of Multiple Indicators of Alzheimer’s Disease Progression. J Prev Alzheimers Dis 10, 212–222 (2023). https://doi.org/10.14283/jpad.2023.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.13