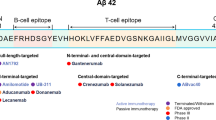
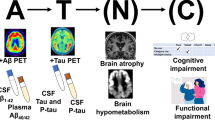
Abstract
Alzheimer’s disease (AD) is a global health concern owing to its complexity, which often poses a great challenge to the development of therapeutic approaches. No single theory has yet accounted for the various risk factors leading to the pathological and clinical manifestations of dementiatype AD. Therefore, treatment options targeting various molecules involved in the pathogenesis of the disease have been unsuccessful. However, the exploration of various immunotherapeutic avenues revitalizes hope after decades of disappointment. The hallmark of a good immunotherapeutic candidate is not only to remove amyloid plaques but also to slow cognitive decline. In line with this, both active and passive immunotherapy have shown success and limitations. Recent approval of aducanumab for the treatment of AD demonstrates how close passive immunotherapy is to being successful. However, several major bottlenecks still need to be resolved. This review outlines recent successes and challenges in the pursuit of an AD vaccine.



Similar content being viewed by others
References
LoGiudice D, & Watson R. Dementia in older people: an update. Internal Medicine Journal 2014; 44(11):1066–1073. https://doi.org/10.1111/imj.12572
Mayeux R, & Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harbor Perspectives in Medicine 2012; 2(8):a006239. https://doi.org/10.1101/cshperspect.a006239
Jha NK, Jha SK, Kar R, Nand P, Swati K & Goswami VK. Nuclear Factor-Kappa β as a therapeutic target for Alzheimer’s disease. J Neurochemistry 2019; 150(2):113–137. doi:https://doi.org/10.1111/jnc.14687
Uversky VN. Intrinsic Disorder in Proteins Associated with Neurodegenerative Diseases. In: Ovádi J., Orosz F. (eds) Protein Folding and Misfolding: Neurodegenerative Diseases. Focus on Structural Biology, vol 2009; 7. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-9434-7_2
Imtiaz B, Tolppanen AM, Kivipelto M, Soininen H. Future directions in Alzheimer’s disease from risk factors to prevention. Biochemical Pharmacology 2014; 88(4):661–670. https://doi.org/10.1016/j.bcp.2014.01.003
Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Archives of Medical Research 2012; 43(8):600–608. https://doi.org/10.1016/j.arcmed.2012.11.003
Alves RP, Yang MJ, Batista MT, Ferreira LC. Alzheimer’s disease: is a vaccine possible?. Brazilian JMedical Biological Research 2014; 47(6):438–444. https://doi.org/10.1590/1414-431x20143434
Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (2012; 8(2):131–168. https://doi.org/10.1016/j.jalz.2012.02.001
Hallock P, Thomas MA. Integrating the Alzheimer’s disease proteome and transcriptome: a comprehensive network model of a complex disease. Omics: AJournal of Integrative Biology 2012;16(1–2):37–49. https://doi.org/10.1089/omi.2011.0054
Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochemical Pharmacology 2014; 88(4): 640–651. https://doi.org/10.1016/j.bcp.2013.12.024
Henderson VW. Alzheimer’s disease: Review of hormone therapy trials and implications for treatment and prevention after menopause. J Steroid Biochem and Mol Biol 2014; 142:99–106. https://doi.org/10.1016/j.jsbmb.2013.05.010
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England Journal of Medicine 2012; 367(9):795–804. https://doi.org/10.1056/NEJMoa1202753
Jack CR, JrKnopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC & Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology 2010; 9(1):119–128. https://doi.org/10.1016/S1474-4422(09)70299-6
Panza F, Lozupone M, Seripa D, Imbimbo BP. Amyloid-β immunotherapy for alzheimer disease: Is it now a long shot?. Annals of Neurology 2019; 85(3):303–315. https://doi.org/10.1002/ana.25410
Haass C. New hope for Alzheimer disease vaccine. Nature medicine 2002; 8(11):1195–1196. https://doi.org/10.1038/nm1102-1195
Schnabel J. Vaccines:Chasing the dream. Nature 2011; 475(7355):S18–S19. doi:https://doi.org/10.1038/475s18a
Sterner RM, Takahashi PY & Yu Ballard AC. Active Vaccines for Alzheimer Disease Treatment. Journal of the American Medical Directors Association 2016; 17(9):862.e11–862.e15. doi:https://doi.org/10.1016/j.jamda.2016.06.009
Alzheimer A, Stelzmann RA, Schnitzlein HN Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eineeigenartigeErkankung der Hirnrinde”. Clinical Anatomy (New York, N.Y.) 1995; 8(6):429–431. https://doi.org/10.1002/ca.980080612
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science (New York, N.Y.) 2002; 297(5580):353–356. https://doi.org/10.1126/science.1072994
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature 2004; 430(7000):631–639. https://doi.org/10.1038/nature02621
Van Gassen G, Annaert W, Van Broeckhoven C. Binding partners of Alzheimer’sdisease proteins: are they physiologically relevant? Neurobiol Dis 2000; 7(3):135–151.
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of Neurology 1999; 45(3):358–368. https://doi.org/10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x
Berg L, McKeel DW, JrMiller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS & Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of Neurology 1998; 55(3): 326–335. https://doi.org/10.1001/archneur.55.3.326
Lambracht-Washington D & Rosenberg RN. Advances in the development of vaccines for Alzheimer’s disease. Discov Med 2013; 15(84):319–326.
Lannfelt L, Relkin NR & Siemers ER. Amyloid-β-directed immunotherapy for Alzheimer’s disease. Journal of Internal Medicine 2014; 275(3):284–295. doi:https://doi.org/10.1111/joim.12168
Jin M, Shepardson N, Yang T, Chen G, Walsh D & Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proceedings of the National Academy of Sciences, U.S.A 2011; 108(14):5819–5824.
Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL & La Ferla FM. Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. The Journal of Biological Chemistry 2006; 281(3):1599–1604. https://doi.org/10.1074/jbc.M507892200
Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ & Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002; 416(6880):535–539. https://doi.org/10.1038/416535a
Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE & Moir RD. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Science Translational Medicine 2016; 8(340):340ra72. https://doi.org/10.1126/scitranslmed.aaf1059
Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L & Larson EB. Dementia and Alzheimer disease incidence: a prospective cohort study. Archives of Neurology 2002; 59(11):1737–1746. https://doi.org/10.1001/archneur.59.11.1737
Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology 1999; 52(1):78–84. https://doi.org/10.1212/wnl.52.1.78
Cruchaga C, Haller G, Chakraverty S, Mayo K, Vallania FL, Mitra RD, et al. Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PloS one 2012; 7(2):e31039. https://doi.org/10.1371/journal.pone.0031039
Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. American Journal of Human Genetics 1999; 65(3):664–670. https://doi.org/10.1086/302553
Wijsman EM, Daw EW, Yu X, Steinbart EJ, Nochlin D, Bird TD & Schellenberg GD. APOE and other loci affect age-at-onset in Alzheimer’s disease families with PS2 mutation. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The official publication of the International Society of Psychiatric Genetics 2005; 132B(1):14–20. https://doi.org/10.1002/ajmg.b.30087
Lopera F, Ardilla A, Martínez A, Madrigal L, Arango-Viana JC, Lemere CA, Arango-Lasprilla JC et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA 1997; 277(10):793–799.
Bretsky PM, Buckwalter JG, Seeman TE, Miller CA, Poirier J, Schellenberg GD, Finch CE & Henderson VW. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Disease and Associated Disorders 1999; 13(4):216–221. https://doi.org/10.1097/00002093-199910000-00007
Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278(16):1349–1356.
Evans CF, Davtyan H, Petrushina I, Hovakimyan A, Davtyan A, Hannaman D, Cribbs DH, Agadjanyan MG & Ghochikyan A. Epitope-based DNA vaccine for Alzheimer’s disease: translational study in macaques. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2014; 10(3):284–295. https://doi.org/10.1016/j.jalz.2013.04.505
van Dyck CH. Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biological Psychiatry 2018; 83(4):311–319. https://doi.org/10.1016/j.biopsych.2017.08.010
Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E & Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nature Neuroscience 2009; 12(12):1567–1576. https://doi.org/10.1038/nn.2433
Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiology of Disease 2004; 15(1):11–20. https://doi.org/10.1016/j.nbd.2003.09.015
Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nature Reviews Immunology 2006; 6(5):404–416. doi:https://doi.org/10.1038/nri1843
Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nature Medicine 2002; 8(11):1270–1275. https://doi.org/10.1038/nm783
Zlokovic BV. Clearing amyloid through the blood-brain barrier. Journal of Neurochemistry 2004; 89(4):807–811. https://doi.org/10.1111/j.1471-4159.2004.02385.x
Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nature Medicine 2003; 9(7):907–913. https://doi.org/10.1038/nm890
Arbel M, Yacoby I, Solomon B. Inhibition of amyloid precursor protein processing by beta-secretase through site-directed antibodies. Proceedings of the National Academy of Sciences U.S.A 2005; 102(21):7718–7723. https://doi.org/10.1073/pnas.0502427102
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999; 400(6740):173–177. https://doi.org/10.1038/22124
Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology 2005; 64(1):94–101. https://doi.org/10.1212/01.WNL.0000148604.77591.67
Zotova E, Bharambe V, Cheaveau M, Morgan W, Holmes C, Harris S, Neal JW, Love S, Nicoll JA & Boche D. Inflammatory components in human Alzheimer’s disease and after active amyloid-β42 immunization. Brain: AJournal of Neurology 2013; 136(Pt 9):2677–2696. https://doi.org/10.1093/brain/awt210
Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, Hock C, Nitsch RM, Masliah E, Growdon JH, Frosch MP & Hyman BT. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain: A Journal of Neurology 2010; 133(Pt 5):1312–1327. https://doi.org/10.1093/brain/awq056
Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C & Nicoll JA. Consequence of Abeta immunization on the vasculature of human Alzheimer’s disease brain. Brain: AJournal of Neurology 2008; 131 (Pt12):3299–3310. https://doi.org/10.1093/brain/awn261
Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interruptedtrial. Neurology 2005; 64(9):1553–1562. https://doi.org/10.1212/01.WNL.0000159740.16984.3C
Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003; 61(1):46–54. https://doi.org/10.1212/01.wnl.0000073623.84147.a8
Ferrer I, Rovira MB, Guerra MLS, Rey MJ & Costa-Jussá F. Neuropathology and Pathogenesis of Encephalitis following Amyloid β Immunization in Alzheimer’s Disease. Brain Pathology 2004; 14(1):11–20. doi:https://doi.org/10.1111/j.1750-3639.2004.tb00493.x
Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW & Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. International Immunology 2003; 15(4):505–514. https://doi.org/10.1093/intimm/dxg049
Wang CY, Wang PN, Chiu MJ, Finstad CL, Lin F, Lynn S, Tai YH, De Fang X, et al. UB-311, a novel UBITh® amyloid β peptide vaccine for mild Alzheimer’s disease. Alzheimer’s & Dementia (New York, N. Y.) 2017; 3(2):262–272. https://doi.org/10.1016/j.trci.2017.03.005
Farlow MR, Andreasen N, Riviere ME, Vostiar I, Vitaliti A, Sovago J, Caputo A, Winblad B & Graf A. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimer’s Research & Therapy 2015; 7(1):23. https://doi.org/10.1186/s13195-015-0108-3
Davtyan H, Bacon A, Petrushina I, Zagorski K, Cribbs DH, Ghochikyan A & Agadjanyan MG. Immunogenicity of DNA- and recombinant protein-based Alzheimer Disease epitope vaccines. Human Vaccines & Immunotherapeutics 2014; 10(5):1248–1255. doi:https://doi.org/10.4161/hv.27882.
Mantile, F., & Prisco, A. Vaccination against β-Amyloid as a Strategy for the Prevention of Alzheimer’s Disease. Biology, 2020; 9(12), 425. doi:https://doi.org/10.3390/biology9120425
Mamikonyan G, Necula M, Mkrtichyan M, Ghochikyan A, Petrushina I, Movsesyan N, Mina E, Kiyatkin A, Glabe CG, Cribbs DH & Agadjanyan MG. Anti-A beta 1–11 antibody binds to different beta-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils but not the most toxic oligomers. The Journal of Biological Chemistry 2007; 282(31):22376–22386. https://doi.org/10.1074/jbc.M700088200
Arai H, Suzuki H, Yoshiyama T. Vanutidecridificar and the QS-21 adjuvant in Japanese subjects with mild to moderate Alzheimer’s disease: results from two phase 2 studies. Curr Alzheimer Res 2015; 12(3):242–254. doi:https://doi.org/10.2174/1567205012666150302154121. PMID: 25731629.
Pasquier F, Sadowsky C, Holstein A, Leterme G, Peng Y, Jackson N, Fox NC, et al. Two Phase 2 Multiple Ascending-Dose Studies of VanutideCridificar (ACC-001) and QS-21 Adjuvant in Mild-to-Moderate Alzheimer’s Disease. Journal of Alzheimer’s Disease: JAD 2016; 51(4):1131–1143. https://doi.org/10.3233/JAD-150376
Hull M, Sadowsky C, Arai H, Le Prince Leterme G, Holstein A, Booth K, Peng Y, Yoshiyama T, Suzuki H, Ketter N, Liu E & Ryan JM. Long-Term Extensions of Randomized Vaccination Trials of ACC-001 and QS-21 in Mild to Moderate Alzheimer’s Disease. Current Alzheimer research 2017; 14(7):696–708. https://doi.org/10.2174/1567205014666170117101537
Godyń J, Jończyk J, Panek D & Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacological Reports: PR 2016; 68(1):127–138. https://doi.org/10.1016/j.pharep.2015.07.006
Kwan, P., Konno, H., Chan, K. Y., & Baum, L. Rationale for the development of an Alzheimer’s disease vaccine. Human Vaccines & Immunotherapeutics. 2019. doi:https://doi.org/10.1080/21645515.2019.1665453
Schneeberger A, Hendrix S, Mandler M, Ellison N, Bürger V, Brunner M, Frölich L, et al. Results from a Phase II Study to Assess the Clinical and Immunological Activity of AFFITOPE® AD02 in Patients with Early Alzheimer’s Disease. The Journal of Prevention of Alzheimer’s Disease 2015; 2(2):103–114. https://doi.org/10.14283/jpad.2015.63
Lemere CA. Immunotherapy for Alzheimer’s disease: hoops and hurdles. Molecular Neurodegeneration 2013; 8(1):36. doi:https://doi.org/10.1186/1750-1326-8-36
Muhs A, Hickman DT, Pihlgren M, Chuard N, Giriens V, Meerschman C, van der Auwera I, van Leuven F, et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proceedings of the National Academy of Sciences (U.S.A.) 2007; 104(23): 9810–9815. https://doi.org/10.1073/pnas.0703137104
Yu YZ & Xu Q. Prophylactic immunotherapy of Alzheimer’s disease using recombinant amyloid-β B-cell epitope chimeric protein as subunit vaccine. Human Vaccines & Immunotherapeutics 2016; 12(11):2801–2804. https://doi.org/10.1080/21645515.2016.1197456
https://www.alzforum.org/therapeutics/aci-24. Last updated 08 October 2020
Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, Maguire RP, et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. The Lancet Neurology 2012; 11(7):597–604. https://doi.org/10.1016/S1474-4422(12)70140-0
Lopez C, Tariot PN., Caputo A, Langbaum JB, Liu F, et al. The Alzheimer’s Prevention Initiative Generation Program: Study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimer’s & dementia (New York, N. Y.), 2019; 5, 216–227. https://doi.org/10.1016/j.trci.2019.02.005
https://www.alzforum.org/news/conference-coverage/picking-through-rubble-field-tries-salvage-bace-inhibitors 20 Dec 2019
Davtyan H, Ghochikyan A, Hovakimyan A, Petrushina I, Yu J, Flyer D, Madsen PJ, Pedersen LO, Cribbs DH & Agadjanyan MG. Immunostimulant patches containing Escherichia coli LT enhance immune responses to DNA- and recombinant protein-based Alzheimer’s disease vaccines. Journal of Neuroimmunology 2014; 268(1–2):50–57. https://doi.org/10.1016/j.jneuroim.2014.01.002
Theunis C, Crespo-Biel N, Gafner V, Pihlgren M, López-Deber MP, Reis P, Hickman DT, et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PloS one 2013; 8(8):e72301. https://doi.org/10.1371/journal.pone.0072301
Grüninger F. Invited review: Drug development for tauopathies. Neuropathology and Applied Neurobiology 2015; 41(1):81–96. https://doi.org/10.1111/nan.12192
Novak P, Zilka N, Zilkova M. et al. AADvac1, an Active Immunotherapy for Alzheimer’s Disease and Non Alzheimer Tauopathies: An Overview of Preclinical and Clinical Development. J Prev Alzheimers Dis 2019;6:63–69. https://doi.org/10.14283/jpad.2018.45
Robinson HL & Pertmer TM. DNA vaccines for viral infections: basic studies and applications. Advances in Virus Research 2000; 55:1–74. https://doi.org/10.1016/s0065-3527(00)55001-5
Martins YA, Tsuchida CJ, Antoniassi P & Demarchi IG. Efficacy and Safety of the Immunization with DNA for Alzheimer’s Disease in Animal Models: A Systematic Review from Literature. Journal of Alzheimer’s Disease Reports 2017; 1(1):195–217. doi:https://doi.org/10.3233/adr-170025
Ghochikyan A, Davtyan H, Petrushina I, Hovakimyan A, Movsesyan N, Davtyan A, Kiyatkin A, Cribbs DH & Agadjanyan MG. Refinement of a DNA based Alzheimer’s disease epitope vaccine in rabbits. Human Vaccines & Immunotherapeutics 2013; 9(5):1002–1010. https://doi.org/10.4161/hv.23875
Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, et al. Reducing AD-Like Pathology in 3×Tg-AD Mouse Model by DNA Epitope Vaccine — A Novel Immunotherapeutic Strategy. PLoS ONE 2008; 3(5):e2124. doi:https://doi.org/10.1371/journal.pone.0002124
Bach P, Tschäpe JA, Kopietz F, Braun G, Baade JK, Wiederhold KH, Staufenbiel M, Prinz M, Deller T, Kalinke U, Buchholz CJ & Müller UC. Vaccination with Abeta-displaying virus-like particles reduces soluble and insoluble cerebral Abeta and lowers plaque burden in APP transgenic mice. Journal of Immunology (Baltimore, Md.:1950) 2009; 182(12):7613–7624. https://doi.org/10.4049/jimmunol.0803366
Petrushina, I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, Patel A, Head E, Cribbs DH & Agadjanyan MG. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. The Journal of Neuroscience:The official Journal of the Society for Neuroscience 2007; 27(46):12721–12731. https://doi.org/10.1523/JNEUROSCI.3201-07.2007
Zou J, Yao Z, Zhang G, Wang H, Xu J, Yew DT & Forster EL. Vaccination of Alzheimer’s model mice with adenovirus vector containing quadrivalent foldable Abeta(1-15) reduces Abeta burden and behavioral impairment without Abeta-specific T cell response. Journal of the Neurological Sciences 2008; 272(1–2):87–98. https://doi.org/10.1016/j.jns.2008.05.003
Lambracht-Washington D, Qu BX, Fu M, Anderson LD, JrEagar TN, Stüve O & Rosenberg RN. A peptide prime-DNA boost immunization protocol provides significant benefits as a new generation Aβ42 DNA vaccine for Alzheimer disease. Journal of Neuroimmunology 2013; 254(1–2):63–68. https://doi.org/10.1016/j.jneuroim.2012.09.008
Kim H-D, Jin J-J, Maxwell JA & Fukuchi K. Enhancing Th2 immune responses against amyloid protein by a DNA prime-adenovirus boost regimen for Alzheimer’s disease. Immunology Letters 2007; 112(1):30–38. doi:https://doi.org/10.1016/j.imlet.2007.06.006
Rosenberg RN, Fu M & Lambracht-Washington D. Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology. Alzheimer’s Research & Therapy 2018; 10(1). doi:https://doi.org/10.1186/s13195-018-0441-4
Davtyan H, Chen WW, Zagorski K, Davis J, Petrushina I, Kazarian K, Cribbs DH, Agadjanyan MG, Blurton-Jones M & Ghochikyan A. MultiTEP platform-based DNA epitope vaccine targeting N-terminus of tau induces strong immune responses and reduces tau pathology in THY-Tau22 mice. Vaccine 2017; 35(16):2015–2024. https://doi.org/10.1016/j.vaccine.2017.03.020
Matsumoto Y, Niimi N & Kohyama K. Development of a New DNA Vaccine for Alzheimer Disease Targeting a Wide Range of Aβ Species and Amyloidogenic Peptides. PLoS ONE 2013; 8(9):e75203. doi:https://doi.org/10.1371/journal.pone.0075203
Xing XN, Zhang WG, Sha S, Li Y, Guo R, Wang C & Cao YP. Amyloid β 3–10 DNA vaccination suggests a potential new treatment for Alzheimer’s disease in BALB/c mice. Chinese Medical Journal 2011; 124(17):2636–2641.
Olkhanud PB, Mughal M, Ayukawa K, Malchinkhuu E, Bodogai M, Feldman N, Rothman S, Lee JH, Chigurupati S, Okun E, Nagashima K, Mattson MP & Biragyn A. DNA immunization with HBsAg-based particles expressing a B cell epitope of amyloid β-peptide attenuates disease progression and prolongs survival in a mouse model of Alzheimer’s disease. Vaccine 2012; 30(9):1650–1658. https://doi.org/10.1016/j.vaccine.2011.12.136
Qu B, Boyer PJ, Johnston SA, Hynan LS & Rosenberg RN. Abeta42 gene vaccination reduces brain amyloid plaque burden in transgenic mice. Journal of the Neurological Sciences 2006; 244(1–2):151–158. https://doi.org/10.1016/j.jns.2006.02.006
Prins ND & Scheltens P. Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future. In Alzheimer’s Research & Therapy 2013; 5(6):56. https://doi.org/10.1186/alzrt220
Panza F, Solfrizzi V, Imbimbo BP, Tortelli R, Santamato A & Logroscino G. Amyloid-based immunotherapy for Alzheimer’s disease in the time of prevention trials: the way forward. In Expert Review of Clinical Immunology 2014; 10(3):405–419. https://doi.org/10.1586/1744666x.2014.883921
Moreth J, Mavoungou C & Schindowski K. Passive anti-amyloid immunotherapy in Alzheimer’s disease: What are the most promising targets? In Immunity & Ageing 2013; 10(1):18. https://doi.org/10.1186/1742-4933-10-18
Morgan D. Immunotherapy for Alzheimer’s disease. In Journal of Internal Medicine 2011; 269(1):54–63). https://doi.org/10.1111/j.1365-2796.2010.02315.x
Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Medicine 2000; 6(8):916–919.
Montoliu-Gaya L & Villegas S. Aβ-Immunotherapeutic strategies: a wide range of approaches for Alzheimer’s disease treatment. Expert Reviews in Molecular Medicine 2016; 18. https://doi.org/10.1017/erm.2016.11
Frost JL, Liu B, Kleinschmidt M, Schilling S, Demuth H-U & Lemere CA. Passive Immunization against Pyroglutamate-3 Amyloid-β Reduces Plaque Burden in Alzheimer-Like Transgenic Mice: A Pilot Study. Neurodegenerative Diseases 2012; 10(1–4):265–270.
Venkataramani V, Wirths O, Budka H, Härtig W, Kovacs GG & Bayer TA. Antibody 9D5 Recognizes Oligomeric Pyroglutamate Amyloid-β in a Fraction of Amyloid-β Deposits in Alzheimer’s Disease without Cross-Reactivity with other Protein Aggregates. Journal of Alzheimer’s Disease 2012; JAD29(2):361–371.
Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A & Grundman M. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Disease and Associated Disorders 2010; 24(2):198–203.
Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, Raskind M, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurology 2012; 11(3):241–249.
Bagyinszky E, Youn YC, An SSA & Kim S. The genetics of Alzheimer’s disease. Clinical Interventions in Aging 2014; 9:535–551.
Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, Grundman M, Liu E & for the AAB-001 201/202 Investigators Effect of Immunotherapy With Bapineuzumab on Cerebrospinal Fluid Biomarker Levels in Patients With Mild to Moderate Alzheimer Disease. Archives of Neurology 2012; 69(8):1002–1010
Sperling RA, Jack CR, JrBlack SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2011; 7(4):367–385. https://doi.org/10.1016/j.jalz.2011.05.2351
Aisen, P.S. Failure After Failure. What Next in AD Drug Development?. J Prev Alzheimers Dis 2019; 6:150. https://doi.org/10.14283/jpad.2019.2
Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, Dowsett SA, Pontecorvo MJ, Dean RA & Demattos R. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2016; 12(2):110–120.
Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM & Paul SM. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nature Neuroscience 2002; 5(5):452–457.
Mably AJ, Liu W, McDonald JM, Dodart JC, Bard F, Lemere CA, O’Nuallain B & Walsh DM. Anti-Aβ antibodies incapable of reducing cerebral Aβ oligomers fail to attenuate spatial reference memory deficits in J20 mice. In Neurobiology of Disease 2015; 82:372–384. https://doi.org/10.1016/j.nbd.2015.07.008
Farlow M, Arnold SE, van Dyck CH, Aisen PS, Joy Snider B, Porsteinsson AP, Friedrich S, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. In Alzheimer’s & Dementia 2012; 8(4):261–271. https://doi.org/10.1016/j.jalz.2011.09.224
Neurology TL & The Lancet Neurology. Solanezumab: too late in mild Alzheimer’s disease? In The Lancet Neurology 2017; 16(2):97. https://doi.org/10.1016/s1474-4422(16)30395-7
Topline Result for First DIAN-TU Clinical Trial: Negative on Primary 10 Feb 2020
https://www.alzforum.org/therapeutics/solanezumab updated May 2020
Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, Fanning K, et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2017; 13(1):8–19.
Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP & Aisen P. The A4 Study: Stopping AD Before Symptoms Begin? Science Translational Medicine 2014; 6(228):228fs13–fs228fs13.
Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, Messer J, Oroszlan K. Gantenerumab: A Novel Human Anti-Aβ Antibody Demonstrates Sustained Cerebral Amyloid-β Binding and Elicits Cell-Mediated Removal of Human Amyloid-β. Journal of Alzheimer’s Disease: JAD 2012; 28(1):49–69.
Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, Ashford E, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Research & Therapy 2017; 9(1):1–15.
High-Dose Gantenerumab Lowers Plaque Load 13 Dec 2017
https://www.alzforum.org/therapeutics/gantenerumab updated 25 January 2021
Ultsch M, Li B, Maurer T, Mathieu M, Adolfsson O, Muhs A, Pfeifer A, Pihlgren M. Structure of Crenezumab Complex with Aβ Shows Loss of β-Hairpin. Scientific Reports 2016; 6:39374.
Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, et al. An Effector-Reduced Anti-β-Amyloid (Aβ) Antibody with Unique Aβ Binding Properties Promotes Neuroprotection and Glial Engulfment of Aβ. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 2012; 32(28):9677–9689.
Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, Ward M, et al. ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 2018; 90(21):e1889–e1897.
Tariot PN, Lopera F, Langbaum JB, Thomas RG, Hendrix S, Schneider LS, Rios-Romenets S, et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimer’s & dementia (New York, N. Y.) 2018; 4:150–160. https://doi.org/10.1016/j.trci.2018.02.002
Porte SLL, La Porte SL, Bollini SS, Lanz TA, Abdiche YN, Rusnak AS, Ho W.H, et al. Structural Basis of C-terminal β-Amyloid Peptide Binding by the Antibody Ponezumab for the Treatment of Alzheimer’s Disease. In Journal of Molecular Biology 2012; 421(4–5):525–536). https://doi.org/10.1016/j.jmb.2011.11.047.
Landen JW, Zhao Q, Cohen S, Borrie M, Woodward M, Billing CB, JrBales K, Alvey C, McCush F, Yang J, Kupiec JW & Bednar MM. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clinical Neuropharmacology 2013; 36(1):14–23.
Topline Results: 18 Months of BAN2401 Might Work 7 Jul 2018
Logovinsky, V., Satlin, A., Lai, R., Swanson, C., Kaplow, J., Osswald, G., Basun, H., & Lannfelt, L. Safety and tolerability of BAN2401—a clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimer’s Research & Therapy 2016;8(1), 14. https://doi.org/10.1186/s13195-016-0181-2
Vellas B, Aisen P. New Hope for Alzheimer’s Disease. J Prev Alzheimers Dis 2021; 8, 238–239. https://doi.org/10.14283/jpad.2021.26
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016; 537(7618):50–56). https://doi.org/10.1038/nature19323.
Aducanumab, Solanezumab, Gantenerumab Data Lift Crenezumab, As Well 10 Aug 2015
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016; 537(7618):50–6. PubMed.
Keep Your Enthusiasm? Scientists Process Brutal Trial Data 16 May 2019
‘Reports of My Death Are Greatly Exaggerated.’ Signed, Aducanumab 24 Oct 2019
Biogen Asks FDA To Approve Aducanumab 8 Jul 2020
Advisory Committee Again Urges FDA to Vote No on Aducanumab 14 Apr 2021
Aducanumab Approved to Treat Alzheimer’s Disease 7 Jun 2021
Szabo P, Mujalli DM, Rotondi ML, Sharma R, Weber A, Schwarz H-P, Weksler ME & Relkin N. Measurement of anti-beta amyloid antibodies in human blood. Journal of Neuroimmunology 2010; 227(1–2):167–174
Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, Younkin S, Younkin L, Schiff R & Weksler ME. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiology of Aging 2009; 30(11):1728–1736.
Imbimbo BP, Ippati S, Ceravolo F, Watling M. Perspective: Is therapeutic plasma exchange a viable option for treating Alzheimer’s disease?. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2020; 6(1):e12004.
Loeffler DA. AMBAR, an Encouraging Alzheimer’s Trial That Raises Questions. Frontiers in Neurology, 2020; 11:459. https://doi.org/10.3389/fneur.2020.00459
Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ. Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol. 2012 Aug;8(8):465–9.
Acknowledgements
The authors would like to express their gratitude to the unknown referees for carefully reading the paper and giving valuable suggestions.
Author information
Authors and Affiliations
Contributions
Author’s contributions: NKJ and SO conceptualized the study and hypotheses. MBU, SB, SR, DK, AA and SKS performed literature search. NKJ draw the schemes and drafted the artwork. GG, DKC, KD, JR and KKK drafted the tables. NKJ, and other authors contributed significantly in editing the manuscript. PK, RKA, PP, SS, VU, FAK, RA, SKJ and MDS significantly contributed during revision. All authors read, edited and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest: The authors declare that they have no conflict of interest.
Consent for publication: All authors have read the final version of the manuscript and have given their consent for publication.
Rights and permissions
About this article
Cite this article
Usman, M.B., Bhardwaj, S., Roychoudhury, S. et al. Immunotherapy for Alzheimer’s Disease: Current Scenario and Future Perspectives. J Prev Alzheimers Dis 8, 534–551 (2021). https://doi.org/10.14283/jpad.2021.52
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2021.52