Abstract
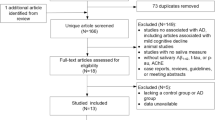
Early AD diagnosis is critical for ameliorating prognosis and treatment. The analysis of CSF biomarkers yields accurate results, but it necessitates a lumbar puncture procedure. Screening for peripheral biomarkers in saliva is advantageous since this medium is noninvasive and inexpensive to obtain. The objective of this systematic review is to analyze saliva biomarker studies which aim to diagnose AD. Titles, abstracts, and reference lists for publications from January 2004 to February 2020 were screened for by searching Google Scholar and PubMed. The inclusion criteria involved published studies that consisted of both AD and control groups. 88 studies were screened, and 20 publications fulfilled the inclusion criteria. These selected publications were scrutinized and included in this review. Aβ42, tau, certain metabolites, and oral microbiota might serve as reliable biomarkers for AD diagnosis. These results showcase the legitimate feasibility of proteomic, metabolomic, and microbiotic compounds in saliva for AD diagnostics in the near future. Supplemental studies must consider standardizing the analytical methods of measuring salivary biomarkers to establish coherence for the selection of valid AD biomarkers. Validation studies will require a large sample size of biomarker-diagnosed individuals for independent populations. This ensures accuracy and rigidity for receiver operating characteristic (ROC) curves that can be set for the most optimal salivary biomarkers in future clinical settings.

Similar content being viewed by others
Availability of Data and Materials
All analyzed and generated data in this study, as well as supplemental information are included in this manuscript.
Abbreviations
- Aβ:
-
Amyloid beta
- Aβ40:
-
Amyloid beta-40 protein
- Aβ42:
-
Amyloid beta-42 protein
- AchE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s Disease
- aMCI:
-
Amnestic mild cognitive impairment
- ANS:
-
Autonomic nervous system
- APP:
-
Amyloid Precursor Protein
- CSF:
-
Cerebrospinal fluid
- EG-ISFET:
-
Extended gate ion-sensitive field-effect transistor
- ELISA:
-
Enzyme-linked immunosorbent-type assay
- FTD:
-
Frontotemporal Dementia
- LC-MS:
-
Liquid chromatography-mass spectrometry
- PD:
-
Parkinson’s Disease
- P-tau:
-
Phosphorylated tau
- ROC:
-
Receiver Operating Characteristic
- SIMOA:
-
Single molecule array
- T-tau:
-
Total tau
- UPLC-MS:
-
Ultra performance liquid chromatography-mass spectrometry
References
Kametani, F., & Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Frontiers in neuroscience, 2018;12, 25. https://doi.org/10.3389/fnins.2018.00025
Honig, L. S., Vellas, B., Woodward, M., Boada, M., Bullock, R., Borne, M., Siemers, E. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. New England Journal of Medicine, 2018;378(4), 321–330. doi: https://doi.org/10.1056/nejmoal705971
National Institute on Aging. What Causes Alzheimer’s Disease. Available from: https://www.nia.nih.gov/health/what-causes-alzheimers-disease.
“Roche provides topline results from investigator-led Phase II/III trial with gantenerumab in rare inherited form of Alzheimer’s disease,” February 2020, https://www.roche.com/media/releases/med-cor-2020-02-10.htm
Farah, R, Haraty, H., Salame, Z., Fares, Y., Qdus, D. M., & Said Sadier, N. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomedical journal, 2018;41(2), 63–87. doi:https://doi.org/10.1016/j.bj.2018.03.004
Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249–254. doi:https://doi.org/10.1038/nature25456
Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647–e1659. doi:https://doi.org/10.1212/WNL.0000000000008081
Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. NatMed. 2020;26(3):387–397. doi:https://doi.org/10.1038/s41591-020-0762-2
Loo, J. A., Yan, W., Ramachandran, P., & Wong, D. T. Comparative human salivary and plasma proteomes. Journal of dental research, 2010;89(10), 1016–1023. doi:https://doi.org/10.1177/0022034510380414
Miklossy, J. Alzheimer’s disease — a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflammation 2011;8, 90. https://doi.org/10.1186/1742-2094-8-90
Tien, N. T., Karaca, I., Tamboli, I. Y., & Walter, J. Trehalose Alters Subcellular Trafficking and the Metabolism of the Alzheimer-associated Amyloid Precursor Protein. The Journal of biological chemistry, 2016;291(20), 10528–10540. doi:https://doi.org/10.1074/jbc.M116.719286
Hill J.M., Clement C, Pogue A.I., Bhattacharjee S., Zhao Y., Lukiw W.J. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD) Front Aging Neurosci. 2014;6:127.
Sayer, R., Law, E., Connelly, P. J., & Breen, K. C. Association of a salivary acetylcholinesterase with Alzheimer’s disease and response to Cholinesterase inhibitors. Clinical Biochemistry, 2004;37(2), 98–104. doi: https://doi.org/10.1016/j.clinbiochem.2003.10.007
Bakhtiari, S., Moghadam, N. B., Ehsani, M., Mortazavi, H., Sabour, S., & Bakhshi, M. Can Salivary Acetylcholinesterase be a Diagnostic Biomarker for Alzheimer?. Journal of clinical and diagnostic research: JCDR, 2017;11(1), ZC58–ZC60. doi:https://doi.org/10.7860/JCDR/2017/21715.9192
Bermejo-Pareja, F., Antequera, D., Vargas, T., Molina, J. A., & Carro, E. Saliva levels of Abetal-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC neurology, 2010;10,108. doi:https://doi.org/10.1186/1471-2377-10-108
Tvarijonaviciute, A., Zamora, C, Cerón, J.J., Bravo-Cantero, A.F., Pardo-Marín, L., Valverde, S., & López-Jornet, P. Salivary biomarkers in Alzheimer’s disease. Clinical Oral Investigations, 2020;1 - 8.
T. Huan, T. Tran, J. Zheng et al., “Metabolomics analyses of saliva detect novel biomarkers of Alzheimer’s disease,” Journal of Alzheimer’s Disease, vol. 65, no. 4, pp. 1401–1416, 2018.
Q. Liang, H. Liu, T. Zhang, Y. Jiang, H. Xing, and A. H. Zhang, “Metabolomics-based screening of salivary biomarkers for early diagnosis of Alzheimer’s disease,” RSC Advances, vol. 5, no. 116, pp. 96074–96079, 2015.
Marksteiner, J., Oberacher, H., & Humpel, C. Acyl-Alkyl-Phosphatidlycholines are Decreased in Saliva of Patients with Alzheimer’s Disease as Identified by Targeted Metabolomics. Journal of Alzheimers Disease, 2019;68(2), 583–589. doi: https://doi.org/10.3233/jad-181278
Bathini, P, Foucras, S, Dupanloup, I, et al. Classifying dementia progression using microbial profiling of saliva. Alzheimer’s Dement. 2020; 12:e12000. https://doi.org/10.1002/dad2.12000
M. Shi, Y. T. Sui, E. R. Peskind et al., “Salivary tau species are potential biomarkers of Alzheimer’s disease,” Journal of Alzheimer’s Disease, vol. 27, no. 2, pp. 299–305, 2011.
H. Pekeles, H. Y. Qureshi, H. K. Paudel, H. M. Schipper, M. Gornistky, and H. Chertkow, “Development and validation of a salivary tau biomarker in Alzheimer’s disease,” Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, vol. 11, pp. 53–60, 2018.
N. J. Ashton, M. Ide, M. Schöll et al., “No association of salivary total tau concentration with Alzheimer’s disease,” Neurobiology of Aging, vol. 70, pp. 125–127, 2018.
M. Lee, J. P. Guo, K. Kennedy, E. G. McGeer, and P. L. McGeer, “A method for diagnosing Alzheimer’s disease based on salivary amyloid-β protein 42 levels,” Journal of Alzheimer’s Disease, vol. 55, no. 3, pp. 1175–1182, 2017.
McGeer, P. L., Lee, M., Kennedy, K., & McGeer, E. G. Saliva Diagnosis as a Disease Predictor. Journal of clinical medicine, 2020;9(2), 377. https://doi.org/10.3390/jcm9020377
C.-B. Kim, Y. Y. Choi, W. K. Song, and K. B. Song, “Antibody based magnetic nanoparticle immunoassay for quantification of Alzheimer’s disease pathogenic factor,” Journal of Biomedical Optics, vol. 19, no. 5, article 051205, 2014.
M. N. Sabbagh, J. Shi, M. Lee et al., “Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: preliminary findings,” BMC Neurology, vol. 18, no. 1, p. 155, 2018.
Liu XX, Jiao B, Liao XX, et al. Analysis of Salivary Microbiome in Patients with Alzheimer’s Disease. J Alzheimers Dis. 2019;72(2):633–640. doi:https://doi.org/10.3233/JAD-190587
H.-C. Lau, I. K. Lee, P. W. Ko et al., “Non-invasive screening for Alzheimer’s disease by sensing salivary sugar using Drosophila cells expressing gustatory receptor (Gr5a) immobilized on an extended gate ion-sensitive field-effect transistor (EGISFET) biosensor,” PLoS One, vol. 10, no. 2, article e0117810, 2015.
Hill J.M., Clement C, Pogue A.I., Bhattacharjee S., Zhao Y., Lukiw W.J. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD) Front Aging Neurosci. 2014;6:127.
E. Carro, F. Bartolomé, F. Bermejo-Pareja et al., “Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin,” Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, vol. 8, pp. 131–138, 2017.
Manni R, Cremascoli R, Perretti C, et al. Evening melatonin timing secretion in real life conditions in patients with Alzheimer disease of mild to moderate severity. Sleep Med. 2019;63:122–126. doi:https://doi.org/10.1016/j.sleep.2019.04.018
Ralbovsky NM, Halámková L, Wall K, Anderson-Hanley C, Lednev IK. Screening for Alzheimer’s Disease Using Saliva: A New Approach Based on Machine Learning and Raman Hyperspectroscopy. J Alzheimers Dis. 2019;71(4):1351–1359. doi:https://doi.org/10.3233/JAD-190675
“Biogen/Eisai Halt Phase 3 Aducanumab Trials I ALZFORUM,” April 2019, https://www.alzforum.org/news/research-news/biogeneisai-halt-phase-3-aducanumab-trials.
Blennow K. A Review of Fluid Biomarkers for Alzheimer’s Disease: Moving from CSF to Blood. Neurology and therapy, 2017;6(Suppl 1), 15–24. doi:https://doi.org/10.1007/s40120-017-0073-9
Dementia, (n.d.). Retrieved from https://www.who.int/news-room/fact-sheets/detail/dementia.
Prvulovic D, Hampel H. Amyloid beta (Abeta) and phospho-tau (ptau) as diagnostic biomarkers in Alzheimer’s disease. Clin Chem Lab Med 2011;49(3): 367–374.
Reale, M., Gonzales-Portillo, I., & Borlongan, C. V. Saliva, an easily accessible fluid as diagnostic tool and potent stem cell source for Alzheimer’s Disease: present and future applications. Brain Research, 2019;146535. doi: https://doi.org/10.1016/j.brainres.2019.146535
Serrano-Pozo, A., Frosch, M. P., Masliah, E., & Hyman, B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine, 2011;1(1), a006189. doi:https://doi.org/10.1101/cshperspect.a006189
Yilmaz, A., Geddes, T., Han, B., Bahado-Singh, R. O., Wilson, G. D., Imam, K., … Graham, S. F. Diagnostic Biomarkers of Alzheimer’s Disease as Identified in Saliva using 1H NMR-Based Metabolomics. Journal of Alzheimers Disease, 2017;58(2), 355–359. doi: https://doi.org/10.3233/jad-161226
Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P, Xu X, et al. Saliva in the diagnosis of diseases. Int J Oral Sci 2016;8(3): 133–37.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MB conducted the literature search, extracted the data, selected the studies for inclusion, analyzed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Not applicable.
Additional information
Consent for Publication
Not applicable.
Competing Interests
The author declares no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Bouftas, M. A Systematic Review on the Feasibility of Salivary Biomarkers for Alzheimer’s Disease. J Prev Alzheimers Dis 8, 84–91 (2021). https://doi.org/10.14283/jpad.2020.57
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2020.57