Abstract
Objectives, Design, Setting
The ketogenic effect of medium chain triglyceride (MCT) oil offers potential for Alzheimer’s disease prevention and treatment. Limited literature suggests a linear B-hyroxybutyrate (BHB) response to increasing MCT doses. This pharmacokinetic study evaluates factors affecting BHB response in three subject groups.
Participants
Healthy subjects without cognitive deficits <65years, similarly healthy subjects >=65years, and those with Alzheimer’s Disease were assessed.
Intervention
Different doses (0g,14g, 28g, 42g) of MCT oil (99.3% C8:0) were administered, followed by fasting during the study period.
Measurements
BHB measured by finger prick sampling hourly for 5 hours after ingestion. Each subject attended four different days for each ascending dose. Data was also collected on body composition, BMI, waist/hip ratio, grip strength, gait speed, nutrient content of pre-study breakfast and side effects.
Results
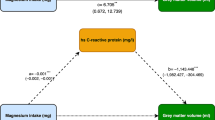
Twenty-five participants: eight healthy; average age of 44yr (25–61), nine healthy; 79yr (65–90) and eight with AD; 78.6yr (57–86) respectively. Compiled data showed the expected linear dose response relationship. No group differences, with baseline corrected area under the blood vs. time curve (r2=0.98) and maximum concentrations (r2=0.97). However, there was notable individual variability in maximum BHB response (42g dose: 0.4 −2.1mM), and time to reach maximum BHB response both, within and between individuals. Variability was unrelated to age, sex, sarcopenic or AD status. Visceral fat, BMI, waist/hip ratio and pretest meal CHO and protein content all affected the BHB response (p<0.001).
Conclusion
There was a large inter-individual variability, with phenotype effects identified. This highlights challenges in interpreting clinical responses to MCT intake.


Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s Disease
- AUC:
-
area under the blood concentration time curve
- BHB:
-
Beta hydroxybutyrate
- BMI:
-
Body Mass Index
- Cmax:
-
maximum blood concentration
- DSM-IV:
-
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- MCT:
-
Medium Chain Triglyceride
- NINCDS-ADRDA:
-
National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
- tmax:
-
time of Cmax
References
Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Paulson OB. Changes in cerebral blood flow and carbohydrate metabolism during acute hyeprketonemia. Am Physiol Soc 1996;270:E746–51
Torosyan N, Sethanandha C, Grill JD, Dilley ML, Lee J, Cummings JL, Ossinalde C, Silverman DH. Chnges in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: results of a randomized, double blind, pilot study. Exp Gerontol 2018;111:118–21
Cunnane SC, Courchesne-Loyer A, Vandenberge C, St-Pierre V, Fortier M, Hennebelle M, et al. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer’s disease. Front Mol Neurosci 2016;9:53
Vandenberghe C, St-Pierre V, Fortier M, Castellano CA, Cuenoud B, Cunnane SC. Medium chain triglycerides modulate the ketogenic effect of a metabolic switch. Front Nutr 2020;7:3
Chatterjee P, Fernando M, Fernando B, Dias CB, Shah T, Silva R, et al. Potential of coconut oil and medium chain triglycerides in the prevention and treatment of Alzheimer’s disease. Mech Ageing Dev 2020;186:111209
McPherson PAC, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem 2012 68:141–151
Caprio M, Infante M, Moriconi E, Armani A, Fabbri A, Mantovani G, Mariani S, Lubrano C, Poggiogalle E, Migliaccio S, et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J Endocrinol Investig 2019 42:1365–1386
Manninen AH. Metabolic effects of the very-low-carbohydrate diets: misunderstood “villains” of human metabolism. J Int Soc Sports Nutr 2004;1:7–11
Ramirez M, Amate L, Gil A. Absorption and distribution of dietary fatty acids from different sources. Early Hum Develop 2001;Suppl 65:S95-S101
Marten B, Pfeuffer M, Schrezenmeir J. Review: Medium-chain triglycerides. Int Dairy J 2006;16:1374–82
Bach AC. Ingenbleek Y, Frey A. The usefulness of dietary medium chain triglycerides in body weight control: fact or fancy? J Lip Res 1996 37(4):708–726
Corchesne-Loyer A, Fortier M, Tremblay-Mercier J, Chouinard-Watkins R, Roy M, Nugent S, et al. Stimulation of mild, sustained ketonemia by medium chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition 2013;29:635–40
Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LES, Reitman ML, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 2016;104:324–33
Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasability and efficacy data form a ketogenic diet intervention in Alzheimer’s disease. Alzh Dement 4:28–36, 2018
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Constantini LC. Study of the ketogenic agent AC1202 in mild to moderate Alzheimer’s Disease: A randomized, double-blind, Placebo controlled trial. Nutr Metab 2009;6:31
Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–46
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC, American Psychiatric Press, 2000
Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing 1997;26:15–9
Juby A, Mager D, Davis C, Jay D, Blackburn T. Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer’s disease: a randomized double-blind placebo cross over study, with an open label extension. Can Geriatr J 2020;23:1;3 (CCD Oral Presentations)
Abbott Laboratories Co 2020 https://www.freestyle.abbott/ca/en/contact.html
Guerci B, Benichou M, Floriot M, Bohme P, Fougnot S, Franck P, Drouin P. Accuracy of an electrochemical sensor for measuring capillary blood ketones by fingerstick samples during metabolic deterioration after continuous subcutaneous insulin infusion interruption in type 1 diabetic patients. Diabetic Care 2003;26(4):1137–41
Forrow NJ, Sanghera GS, Walters SJ, Watkin JL. Development of a commercial amperometric biosensor electrode for the ketone D-3-hydroxybutyrate. Biosens Bioelectron 2005;20(8):1617–25
Government of Canada, Clinical trials for Natural health products, Appendix 8:Adverse Reaction report form for Clinical trials, https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/guidance-documents/clinical-trials.html
Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill Jr GF. Brain metabolism during fasting. J Clin Invest 1967;46:1589–95
Cahill Jr GF, Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc 2003;114:149–61
Buchhalter JR, D’Alfonso S, Connolly M, Fung E, Michoulas A, et al. The relationship between D-beta-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open 2017;2:317–321
Urbain P, Bertz H. Monitoring for compliance with a ketogenic diet: what is the best time of day to test for urinary ketosis? Nutr Metab 2016 13:77–8
Volek JS, LaFountain RA, Dituro P. Extended ketogenic diet and physical training in military personnel. Military Med 2019;184:199–200
Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing Type 2 diabetes: a narrative review of the evidence. Nutrients 2019;11:766
Phinney SD, Bistrian BR, Evans JW, Gervino E, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabol 1983;32:769–776
Brocks DR, Davies NM. Lymphatic drug absorption via the enterocytes: pharmacokinetic simulation, modeling, and considerations for optimal drug development. J Pharm Pharm Sci 2018;21:254s–270s
Griffith ML, Younk LM, Davis SN. Visceral adiposity, insulin resistance, and Type 2 Diabetes. Am J Lifestyle Med 2010;4:230–243
Willette AA, Bendlin BB, Starks BS, Birdsill AC, Johnson SC, Christian BT, Okonkwo OC, La Rue A, Hermann BP, Koscik RL, et al. Association of insulin resistance with cerebral glucose uptake in late-middle aged adults at risk for Alzheimer’s disease. JAMA Neurol 2015;72(9):1013–1020
McDonald TJW, Cervenka MC. The expanding role of ketogenic diets in adult neurological disorders. Brain Sci 2018;8:148
Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, Ho M, Roberts A, Robertson J, VanItallie TB, Veech RL. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 2012;63:401–408
Shivva V, Cox PJ, Clarle K, Veech RL, Tucker IG, Duffull SB. The population pharmacokinetics of D-B-hydroxybutyrate following administration of (R)-3-3hydroxybutyrate (R)-3-hydroxybutyrate. The AAPS J 2016 18(3):678–688
Freemantle E, Vandal M, Tremblay-Mercier J, Plourde M, Poirier J, Cunnane SC. Metabolic response to a ketogenic breakfast in the healthy elderly. J Nutr, Health, Aging. 2009 13:293–298
Vandenberghe C, St-Pierre V. Perotti T, Fortier M, Castellano CA, Cunnane SC. Tricaprylin alone increases plasma ketone response more than coconut oil or other medium-chain triglycerides: an acute crossover study in healthy adults. Curr Dev Nutr 2017;1:1–5
Courchesne-Loyer A, Lowry CM, St-Pierre V, Vandenberghe C, Fortier M, Castellano CA, et al. Emulsification increases the acute ketogenic effect and bioavailability of medium-chain triglycerides in humans. Curr Dev Nutr 2017;1:e000851
Vandenberghe C, Castellano CA, Maltais M, Fortier M, St-Pierre V, Dionne J, Cunnane SC. A short term intervention combining aerobic exercise with medium chain triglycerides (MCT) is more ketogenic than either MCT or aerobic exercise alone: a comparison of nomoglycemic and prediabetic older women. Appl Physiol Nutr Metab 2019;44:66–73
Acknowledgements
The participants who gave up their time to be in the study. Vickie Baker (Registered Nurse) who assisted with data collection. Dr Vera Mazurak’s lipid research laboratory for independently verifying the triglyceride content of the test oil.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributions of Authors
AGJ: designed the research, conducted the research, analyzed data, wrote the paper and had primary responsibility for the final content. DRB was involved in study design, and performed the pharmacokinetic statistical analysis. DAJ was involved in conducting the research, and data analysis. CMJD was involved in conducting the research, and data analysis. DRM performed the non-pharmacokinetic statistical analysis. All authors read and approved the final manuscript.
Conflict of Interest
Angela G Juby, Dion R Brocks, David A Jay, Christopher MJ Davis, Diana R Mager, all have no conflicts of interest with respect to this study.
Rights and permissions
About this article
Cite this article
Juby, A.G., Brocks, D.R., Jay, D.A. et al. Assessing the Impact of Factors that Influence the Ketogenic Response to Varying Doses of Medium Chain Triglyceride (MCT) Oil. J Prev Alzheimers Dis 8, 19–28 (2021). https://doi.org/10.14283/jpad.2020.53
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2020.53