Abstract
Background
Frailty in older adults is a rapidly growing unmet medical need. It is an aging-related syndrome characterized by physical decline leading to higher risk of adverse health outcomes.
Objectives
To evaluate the efficacy of Lomecel-B, an allogeneic medicinal signaling cell (MSC) formulation, in older adults with frailty. DESIGN: This multicenter, randomized, parallel-arm, double-blinded, and placebo-controlled phase 2b trial is designed to evaluate doserange effects of Lomecel-B for frailty on physical functioning, patient-reported outcomes (PROs), frailty status, and biomarkers.
Setting
Eight enrolling clinical research centers, including the Miami Veterans Affairs Medical Center.
Participants
Target enrollment is 150 subjects aged 70–85 years of any race, ethnicity, or gender. Enrollment criteria include a Clinical Frailty Score of 5 (“mild”) or 6 (“moderate”), a 6MWT of 200–400 m, and serum tumor necrosis factor-alpha (TNF-α) ≥2.5 pg/mL.
Intervention
A single intravenous infusion of Lomecel-B (25, 50, 100, or 200 million cells) or placebo (N=30/arm). Patients are followed for 365 days for safety, and the efficacy assessments performed at 90, 180, and 270 days.
Measurements
The primary endpoint is change in 6MWT in the Lomecel-B-treated arms versus placebo at 180 days post-infusion. Secondary and exploratory endpoints include change in: 6MWT and other physical function measures at all time points; PROs; frailty status; cognitive status; and an inflammatory biomarkers panel. A pre-specified sub-study examines vascular/endothelial biomarkers. Safety is evaluated throughout the trial.
Results
The trial is conducted under a Food and Drug Administration Investigational New Drug (IND), with Institutional Review Board approval, and monitoring by an NIH-appointed independent Data Safety Monitoring Board.
Conclusion
This clinical trial investigates the use of a regenerative medicine strategy for frailty in older adults. The results will further the understanding of the potential for Lomecel-B in the geriatric condition of frailty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty is an increasingly prevalent age-related multidimensional syndrome, which manifests with heterogeneous physical and cognitive symptoms rendering affected older adults at higher risk for adverse health outcomes and substantial socioeconomic consequences (1, 2). Due to the high prevalence of frailty in older adults and the lack of approved medical treatments or standards of care, developing appropriate therapies for frailty represents an important unmet medical need (3, 4).
Mechanistically, inflammaging, which is defined as an age-associated low-grade chronic inflammatory state (5), reduced regenerative capacity (6), and vascular endothelial dysfunction (7) are among the important biological underpinnings of the pathophysiology of frailty (8). Countering these pathological pathways presents a potential therapeutic approach and warrants the development of novel therapeutic strategies. There has been a significant increase in investigation of cell-based therapies for age-related chronic conditions including physical frailty and cognitive impairment. Medicinal signaling cells, also known as mesenchymal stem/stromal cells (MSCs), are multipotent culture-expanded cells with pleiotropic mechanisms of action (9, 10). MSCs have cellular and humoral immunomodulatory and pro-vascular properties, migrate to sites of inflammation and injury, regulate host stem cell niches through paracrine effects and heterocellular coupling, and can stimulate inherent regenerative and reparative mechanisms (7, 9, 10). Moreover, MSCs are immunoprivileged/immunoevasive, and are not subject to rejection by the receiving host, allowing the use of allogeneic products that have well-documented safety profiles (11).
The first human trial investigating the application of allogeneic MSCs in older adults with frailty was the CRATUS study that demonstrated acceptable safety and tolerability results of allogeneic MSCs (12, 13). The CRATUS study also provided promising efficacy findings which allowed the powering of the current study to be determined. The phase I CRATUS trial was an open label study investigating safety and efficacy of a single intravenous infusion of allogeneic MSCs (20, 100, or 200 million cells/infusion, N=5 patients/arm). This study met its primary safety objective, and no treatment-emergent serious adverse events (TE-SAEs) were attributed to the study intervention. Moreover, the six-minute walk test (6MWT) distance (6MWD) increased at 3- and 6-months post-treatment in the allogeneic MSC groups (up to 76.6 m from baseline in Phase 1, and 64.8 m in Phase 2), and circulating TNF-α levels decreased at 6 months after infusion in all treatment groups. The randomized placebo-controlled phase 2 portion of CRATUS examined safety of 100 or 200 million allogeneic MSCs versus placebo in 30 frail participants (N=10/arm). In addition, this study was designed to obtain provisional efficacy data to inform a larger next-stage trial powered for efficacy. This study supported the excellent safety profile of allogeneic MSC infusions in frail older individuals, with no TE-SAEs or other safety concerns, meeting the primary endpoint. Additionally, the Phase 2 results were consistent with the results from Phase 1 in that the allogeneic MSC groups showed improvements in 6MWT, respiratory function, female sexual quality of life, and serum TNF-α. These findings provided support for further investigation of allogeneic MSCs as a therapeutic strategy for frail older adults.
As the next-step in this program, we designed the randomized, double-blinded and placebo-controlled phase 2b trial presented here. This trial is powered to detect changes in 6MWT, and further designed to evaluate dose-response to an allogeneic MSC formulation, called Lomecel-B, in the target population. The subject population consists of older adults with mild to moderate physical frailty, as determined by: age; clinically-assessed mild to moderate frailty; a 6MWD of 200–400 meters, which is supportive of the frailty status; and whom had an underlying pro-inflammatory state, a characteristic of frailty (5). The subject population is also cognitively intact to minimized related confounding issues, e.g., inability to follow assessment instructions. Study powering is based on the 6MWT, used as the primary endpoint. The trial is designed to evaluate changes in important indicators of frailty status in the domains of physical functioning, patient-reported outcomes (PROs), biomarkers, and frailty status. We hope to objectively gain important insights into the use of Lomecel-B as a biological therapy to improve health status in those with frailty.
Methods
Trial Design
The title of this trial is “A Phase 2b, Randomized, Blinded and Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Longeveron Allogeneic Human Mesenchymal Stem Cells Infusion in Patients with Aging Frailty”, and is registered with ClinicalTrials.gov (NCT03169231). This Phase 2b trial is double-blinded, randomized, and placebo-controlled with parallel arms at doses ranging from 0 to 200M cells of Lomecel-B. Oversight is by a single IRB (Western IRB: Puyallup, WA), independent pharmacovigilance group (ProPharma Group: Washington, DC), independent DSMB, and independent clinical monitors (Syneos/Joulé Inc.: Edison, NJ). The study and manufacturing of investigational product are under Food and Drug Administration (FDA) oversight as an Investigational New Drug Application (IND). IQVIA (Durham, NC) is selected as the CRO for this study.
Target enrollment is 150 males and females aged 70–85, who provide written informed consent, with a Clinical Frailty Score of 5 (“mild”) or 6 (“moderate”) (14), a six minute walk test (6MWT) of 200–400 meters, a score ≥ 24 on the Mini Mental Status Examination (MMSE), and a serum tumor necrosis factor-alpha (TNF-α) of ≥ 2.5 pg/mL at screening. The rationale for these selection criteria is detailed in the Discussion section.
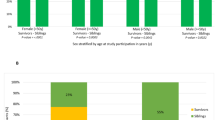
The complete inclusion and exclusion criteria are listed in Table 1. Subjects are randomized (1:1:1:1:1) within each investigational site to receive a single intravenous infusion of 25, 50, 100, or 200 million Lomecel-B, or Placebo (N=30/arm) as shown in Figure 1. The infusion day is defined as Day 0. Safety and efficacy assessments are conducted at 30, 90, 180, and 270 days after infusion. A follow up telephone call at 12 months post-infusion is performed for clinical outcomes and adverse event data collection.
Target enrollment is 150 subjects aged 70–85 years of any race, ethnicity, or gender. Enrollment criteria includes a Clinical Frailty Score of 5 (“mild”) or 6 (“moderate”), a six-minute walk test (6MWT) of 200–400 m, a Mini Mental Status Examination (MMSE) score ≥24, and a serum tumor necrosis factor-alpha (TNF-α) level of ≥2.5 pg/mL. Eligible subjects are randomized (1:1:1:1:1) to receive a single intravenous infusion of 25, 50, 100, or 200 million Lomecel-B, or Placebo (N=30/arm). The infusion day is defined as Day 0. Safety and efficacy assessments are conducted at 30-, 90-, 180-, and 270-days post-infusion. A follow-up telephone call at 12 months post-infusion is performed for clinical outcomes and adverse event data collection.
Lomecel-B and Placebo
Lomecel-B is a formulation of allogeneic MSCs, prepared by Longeveron under an FDA IND Chemistry, Manufacturing, and Controls (CMC) section. The starting material is sourced from healthy young adult donors in compliance with the Code of Federal Regulations, specifically 21 CFR part 1271, and culture-expanded using current Good Manufacturing Practices (cGMP). The placebo is the vehicle used for resuspension of Lomecel-B (PlasmaLyte-A with 1.0% human serum albumin). Lomecel-B and placebo are prepared in identically appearing infusion bags bearing identical appearing labels and delivered via peripheral intravenous infusion in an out-patient setting.
Study Measures and Outcomes
A timetable of the study assessments and activities is presented in Table 2.
Efficacy Measures
Clinical assessments for efficacy are performed at baseline, and at 90-, 180- and 270-days post infusion, except for the MMSE which is only performed at the screening visit as an inclusion criterion and at 180 days post-infusion. Additionally, a CSHA Clinical Frailty Scale assessment and 6MWT are also performed at the screening visit as inclusion criteria in addition to the other visits. These assessments include:
Physical Function/Performance Assessments:
-
The Six-Minute Walk Test (6MWT) serves as the primary endpoint of the trial, using the administration guidelines established by the American Thoracic Society (15). The 6MWT measures the distance the subject can walk in six minutes, and is an integrated measure of physical capacity and mobility (16). Another well established and commonly used physical function measurement is the 400m walk test (17), however the 6MWT is designed to stress the cardiorespiratory capacities to estimate the physiological reserves and exercise tolerance. It measures the capacity to cope with challenging and prolonged stressors.
-
Grip strength, tested via dynamometer, is a validated diagnostic marker of frailty with high sensitivity, specificity, and accuracy (18, 19). Both dominant and non-dominant hands are measured.
-
The Short Physical Performance Battery (SPPB) is an objective assessment of balance, lower body strength, and mobility (20, 21). It entails a 4-Meter Walk Gait Speed Test, five chair-stand test, and a balance test. The SPPB is predictive of important health outcomes in older adults, including disability, hospitalization, institutionalization, and mortality (17).
-
The Tinetti-Performance Oriented Mobility Assessment (POMA) assesses gait and balance ability, and is used to evaluate a person’s risk for falling within the next year (22). Higher scores indicate better the performance, to a maximum score of 28 points. In general, scores < 19 points indicate a high risk for falling. POMA scores are significantly lower in frailty, and can differentiate older adults with frailty from those who are pre-frail (23).
-
Forced Expiratory Volume in the first second (FEV1) is measured by spirometry to evaluate respiratory capacity. Reduced FEV1 correlates with reduced gait speed in older adults with mobility limitations (24).
Frailty Assessments:
-
The Canadian Study of Health and Aging (CSHA) derived and validated Clinical Frailty Scale (CFS) is an instrument for measuring frailty status based on clinical judgment (14). The instruments ranks the subject between a CFS of 1 (very fit) to 9 (terminally ill). In this study, enrolled subjects must have a screening CFS of 5 (mildly frail) or 6 (moderately frail).
-
Frailty Phenotype Assessment (FPA) using Cardiovascular Health Study (CHS) criteria is an operationalized phenotype definition developed and validated by Fried and colleagues (4). The CHS Frailty Phenotype is based on five criteria for evaluating specific signs and symptoms associated with frailty that are often slightly modified for differences in population, geographic region, and physiology (25–28). In this trial, the specific criteria evaluated are as follows:
-
Unintentional weight loss of greater than 10 pounds or more than 5% of body weight in the past year (as self-reported at screening for baseline) or compared to baseline at follow-up visits.
-
Endurance and energy measured by self-reported exhaustion and fatigue based on the following two questions from Center for Epidemiologic Studies Depression Scale (CES-D) (29). A score of “1” is given if responses to both questions are either “moderate amount of time” or “most of the time”. A score of “0” is given if the responses to both questions are either “rarely or none of the time” or “some or little of the time”:
-
-
I felt that everything I did was an effort in the last week:
-
Rarely or none of the time (<1 day)
-
Some or little of the time (1 to 2 days)
-
Moderate amount of the time (3 to 4 days)
-
Most of the time
-
-
I could not get going in the last week:
-
Rarely or none of the time (<1 day)
-
Some or little of the time (1 to 2 days)
-
Moderate amount of the time (3 to 4 days)
-
Most of the time
-
-
Physical activity level as self-reported in the past 3 month: weight bearing physical activity was not performed, more than four hours per day were spent sitting, and went for a short walk once per month or less (Adapted from (30, 31)).
-
Weakness as measured by an average dominant hand grip strength of ≤30 kg for men and ≤18 kg for women in the grip strength test (Adapted from (30)).
-
Slowness as assessed by a time of ≥ 6 seconds in the 4-meter gait speed test (performed as part of the SPPB assessment).
For scoring purposes, in each of these five subsections a score of 0 (subject did not meet the specific criterion) or 1 (subject met the specific criterion) is assigned, and then the final frailty score is obtained as the sum of all the five items. Accordingly, this generates a 6-level ordinal variable ranging from 0 to 5, which is categorized into a 3-level variable representing robustness (none of the criteria are met), pre-frailty (one or two criteria are met) and frailty (3 or more criteria are met).
PROs and Quality-of-Life (QOL) assessments:
-
The Patient-Reported Outcomes Measurement Information System (PROMIS) is a set of validated measures for evaluating physical, mental and social health in adults and children across all conditions (32). Each questionnaire yields a summed raw score, which is then converted into standardized T-scores. The adult PROMIS Physical function—Short Form 20a was selected as a secondary endpoint in this study since it has shown strong test-retest reliability, and showed a minimally important difference of 2 points (~0.20 SD) (33). The adult PROMIS Mobility and PROMIS Upper Extremity are used as exploratory PROs.
-
The Falls Efficacy Scale-International (FES-I) is a short, easy to administer tool that measures the level of concern about falling on a four-point Likert scale (1=not at all concerned to 4=very concerned (34). The FES-I was developed in a collaborative effort with members of the Prevention of Falls Network Europe (ProFaNE), European Committee focused on fall prevention and the psychology of falling. The FES-I has excellent internal validity (Cronbach’s alpha=0.96) and test-retest reliability (ICC=0.96).
-
Geriatric Depression Scale Short Form (GDS-SF) is a 15-item screening tool used to identify depression in older adults. Higher GDS-SF scores indicating increased depression, are seen in older adults with frailty compared to individuals who are pre-frail (23).
-
Sexual quality of life is measured by the Sexual Quality of Life-Female (SQOL-F) (35) and International Index of Erectile Function (IIEF) (36). These measure are chosen as sexual functioning is reduced, and related stress is increased, in older adults with frailty (37).
Cognitive Assessments:
-
To evaluate global cognitive function the Mini Mental State Exam (MMSE) and Montreal Cognitive Assessment (MoCA) are performed to assess the cognitive component of frailty (23). The MMSE is also part of the inclusion criteria.
Safety Measures
Safety assessments are conducted throughout the trial as described in the schedule of events (Table 2) as follows. Blinded attributions of any potential changes to the infusion product is made by the clinical investigator.
-
Physical examination: Vital signs are assessed at each study visit at least once, including weight; height; temperature; heart rate; blood pressure; respiratory rate; pulse oximetry; general appearance; skin and nails; limbs; lymph nodes; head, eyes, ears, nose, throat, and neck; respiratory; cardiovascular; and abdominal.
-
Electrocardiogram (ECG): 12-lead ECG is to assess for any clinically significant cardiac changes over the course of the trial.
-
Laboratory tests: Hematology, blood chemistry, coagulation, and urinalysis are to assess for any significant laboratory changes over the course of the trial. Shift tables in each category are generated and reviewed by the Medical Monitor to capture clinically significant changes.
-
Anti-HLA allo-antibody production: Panel reactive antibody (PRA) tests are used to for signs of graft rejection.
-
Medical history: Comprehensive past, social, and family history is obtained at the screening visit. The medical history is obtained via an interview and by medical records.
-
All adverse events and serious adverse events occurring at any time during the trial will be collected, documented, and reported by the investigator. For each event, the investigator will provide the date of onset and resolution, intensity, treatment required, outcome, seriousness, and potential causality with regards to the study product or infusion procedure.
-
Concomitant medications: Current medications, including prescription and over-the-counter medications, are recorded.
Laboratory Evaluations and Biomarkers
Blood (Screening, Day 0, and at each follow-up) and urine (Screening, and at each study visit post-infusion) samples collection is for safety and efficacy evaluations. As part of the enrollment criteria, serology for communicable diseases is performed at screening visit. High-sensitivity immunoassays are performed for quantification of blood-based biomarkers including: interleukin (IL)-1α, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α, interferon-gamma (IFN-γ), Vascular Endothelial Growth Factor (VEGF)-A, VEGF-C, VEGF-D, VEGF receptor 1 (VEGFR-1), D-dimer, basic Fibroblast Growth Factor (bFGF), placental growth factor (PlGF), Tie-2, and Transforming growth factor-β (TGF-β). Q2 Solutions (Morrisville, NC) was contracted as the independent central laboratory for this study.
Study Endpoints
Primary Efficacy Endpoint
The primary efficacy endpoint is the change from baseline in 6MWT compared to placebo at 180 days post-infusion. The primary endpoint is analyzed in two ways. First, an analysis is performed to examine whether any individual dose of Lomecel-B differs from placebo. Second, an analysis assesses for the presence of a dose-response effect between amount of cell product infused and distance walked.
Secondary Efficacy Endpoints
The secondary endpoints are the following compared to placebo at 180 days post-infusion.
-
1.
Change in PROMIS—Physical Function—Short Form 20a total score
-
2.
Change in serum TNF-α
Exploratory Endpoints
The key exploratory endpoints expand upon the primary and secondary endpoints. First, the primary endpoint analysis is expanded to evaluate whether there is a difference between active Lomecel-B and placebo at any or across all time-points, and whether a dose-response curve is evident at other time points. Second, the secondary endpoint analysis is expanded for the PROMIS questionnaire to explore other questionnaires. Changes in patient self-reported outcomes will be correlated with changes in 6MWT. Thirdly, biomarker analysis is explored using a panel of inflammatory and vascular-endothelial biomarkers.
Exploratory analysis covers changes across visits for all other clinical assessments not included in the list of primary and secondary endpoints, and blood-based biomarkers.
In addition, a pre-specified sub-study evaluates biomarkers of the metabolic syndrome. These include glucagon, leptin, C-peptide, gastric inhibitory polypeptide (GIP) (active), glucagon-like peptide 1 (GLP-1) (active), insulin, and pancreatic polypeptide (PP).
Clinical events
The incidence of the following clinical outcomes is assessed through 365 days post-infusion: falls, fractures, admissions to healthcare facility (e.g., assisted-living facility, nursing home, long-term care facility, etc.), hospitalizations, and death.
Sample Size and Statistical Analysis
Statistical analysis is performed by an independent party (Pharma Data Associates, NJ). The sample size calculation is based on the primary endpoint, the change from baseline in 6MWT at 6 months (A6MWT). Thirty (30) subjects per treatment arm provides approximately 80% power to demonstrate an effect size of 0.75 (treatment difference of each dose vs placebo in change from baseline in 6MWT divided by the common standard deviation) using a one-sided α=0.025. Assuming the common SD of 75 m, this sample size provides 50% and 80% power for the between treatment difference of 39 m and 56 m, respectively. These distances exceed prior calculated minimal clinically important differences (see Discussion) and are less than the changes seen in the CRATUS study (up to 76.6 m) (12, 13).
The analysis of the primary efficacy endpoint is performed on the modified intent to treat (MITT) population which includes all randomized subjects who have received an infusion and have at least one post-baseline assessment for the primary efficacy endpoint. Each dose group is compared to the placebo group in pairwise comparisons using a Mixed-Effect Model Repeated Measure (MMRM) method. The treatment effect is analyzed using MMRM including change from baseline at each post-treatment time point up to month 9 as the response variable, treatment, visit, interaction between treatment and visit as fixed factors, baseline distance as covariate, and patient as repeated measure unit. An unstructured (US) variance-covariance matrix is used to model the correlation among repeated measurements. To account for multiple testing of the different dose groups versus placebo, the step-up Hochberg (1988) procedure is used for the primary analysis of the primary endpoint. For the secondary analysis of the primary endpoint (dose-response effect), MCP-Mod (multiple comparison procedure - modeling) method is used.
The MCP-Mod method is a hybrid approach combining hypothesis testing and modeling in a structured manner to analyze phase 2 dose-ranging studies with the purpose of finding suitable dose(s) for confirmatory phase 3 trials (38, 39). The first step of the procedure (MCP-step) is used to assess presence of a dose-response signal using a trend test deducted from a set of pre-specified candidate models. The second step (Mod-step) relies on parametric modeling or model averaging to find the “optimal” dose for confirmatory trials.
In the first step, linear, quadratic, Emax and Sigmoid Emax dose-response models are used for the trend test in A6MWT. In the second step, the model mean of the dose-response curve is plotted with 95% confidence interval.
Statistical testing for the key secondary endpoint PROMIS—Physical Function—Short Form 20a and all other secondary/exploratory endpoints uses MMRM method without adjusting for multiple testing. The Fisher’s exact test is used to analyze subject incidences of falls, fractures, admission to healthcare facility, hospitalizations, and deaths.
To assess the relationship between outcome variables, correlations are calculated for the absolute values as well as the changes from baseline between the primary endpoint 6MWT and the secondary/exploratory PRO endpoints, and other biomarkers. In addition, regression analyses are conducted to evaluate whether these specific secondary/exploratory endpoints and PRO instruments can predict the clinical outcome measured as 6MWT in this population.
Safety assessments are based mainly on the nature, frequency, relationship, and severity of adverse events (AEs). AEs are coded by primary system organ class (SOC) and preferred term (PT) according to the Medical Dictionary for Regulatory Activities. The treatment-emergent adverse events (TE-AEs) are summarized by the number and percentage (n and %) of subjects in each SOC and PT. For summaries by relationship to study drug and relationship to study infusion, “Definitely/Probably/Possibly related” are combined, and “Unlikely/Unrelated” are combined. When multiple AEs are reported with the same preferred term, the AE of the strongest relation is included in summary by relationship, and the AE of the most severe grade is included in the summary by severity table.
Discussion
This study is designed to assess the effect of 4 doses of Lomecel-B compared to placebo on mobility and exercise tolerance, patient-reported physical function, and biomarkers for inflammation and vascular-endothelial function, in older adults with frailty. Frailty is a common and important geriatric syndrome characterized by age-associated declines in physiologic reserve and function across multi-organ systems, leading to increased vulnerability to stressors and a higher risk for adverse health outcomes (40). When exposed to stressors such as acute or chronic illness (e.g. myocardial infarction), a new drug, a “minor” infection, or iatrogenically (e.g. surgery), frail patients are at risk for marked and often disproportionate decompensation, adverse events, procedural complications, prolonged recovery, functional decline, disability and mortality (4, 40–44).
Proposed factors underlying the biology of frailty include chronic systemic inflammation (45–47), vascular-endothelial dysfunction, endocrine dysfunction, neuropsychological impairment, cardio- and cerebrovascular disease, and malnutrition (44, 48, 49). In frailty, muscle breakdown exceeds muscle synthesis, leading to a progressive decline in muscle mass, strength, and function, and sarcopenia. Under stressed conditions, subclinical impairments are unmasked, and a vicious cycle ensues with physical inactivity and malnutrition leading to further decline. The clinical manifestations of frailty can present as a constellation of signs and symptoms, which can vary to a degree among individuals (4, 41, 42). Common clinical indicators of frailty are sarcopenia, fatigue, weakness, exhaustion, poor endurance, low physical activity, poor balance, slow gait speed, falls, anorexia, malnutrition, and weight loss. Improving mobility and physical functionality may result either concurrently or subsequently in improvements in other associated signs and symptoms.
Study Population
The study population for this trial consists of older adults with mild to moderate frailty. The choice of this population was informed in part by results from the CRATUS trial (12, 13), and improved study powering by reducing baseline variability, given the relatively modest sample size. The CRATUS study enrolled subjects with CFS scores ranging from 4 (“vulnerable”) to 6 (“moderate frailty”). In CRATUS study, it was shown that those with a CFS score of 4 performed relatively well on the 6MWT (most >400 m) and raised concerns that this could impose a ceiling effect on potential improvement in more robust patients, and thereby confound interpretation of the primary endpoint if such patients were included in this study (Oliva et al., unpublished). On the other hand, we were also concerned that subjects with more severe frailty (CFS score ≥ 7) might not respond as effectively to a single infusion of Lomecel-B, which again could confound the data. From our analyses of CRATUS, we found that 83% of subjects with baseline CFS scores ≥ 5 (i.e., frail) had 6MWT distances < 400 m, and 85% of those subjects with baseline 6MWT distances >400 m had CFS scores < 5 (i.e., predisposition to frailty). Most subjects with a CFS score or 5–6 also had a 6MWD >200 m. Thus, we narrowed the population to those with a 6MWD of 200–400 m, which would be mutually confirmatory of the clinician-assessed CFS score of 5 – 6.
The 6MWT has a documented learning curve that is effectively overcome after 2 trials (50). Thus, 6MWT is performed twice at screening to overcome this learning curve that might otherwise be misconstrued as placebo-effect. The third 6MWT, performed at baseline, acts as the formal baseline measure for this study.
Frailty in older adults is underlined by a chronic low-level pro-inflammatory state, in which other groups have found significant correlations to serum levels of TNF-α and other pro-inflammatory cytokines (5). In CRATUS, we found that subjects with baseline serum TNF-α ≥ 2.5 pg/mL appeared to have greater responses to a single dose of cells (unpublished results) compared with those below 2.5 pg/mL. We thus incorporated a serum TNF-α ≥ 2.5 pg/mL as part of the enrollment criteria to capture a potentially more responsive patient population. Given the anti-inflammatory potential of Lomecel-B, regular use of TNF-α antagonists or powerful steroidal anti-inflammatory medication (e.g., Prednisone) is an exclusion criteria to reduce potential confounding issues introduced by such medications.
Finally, the trial incorporates exclusion criteria intended to minimize confounding effects of severe comorbidities. These include exclusions for poorly controlled diabetes or hypertension, advanced hepatic, cardiac, pulmonary, or renal disease. Exclusions are also made for neurological conditions and cognitive disorders (e.g., dementia), to enrich for a population with physical, but not cognitive, frailty. Subjects with any physical disability that would impact their mobility are also excluded.
Dosing Selection Rationale
To gain clarity on potential optimal dosing, this Phase 2b trial is designed to evaluate dose-response using dosages that double from 25M to 200M cells, versus placebo. This dose range was chosen based on provisional efficacy data from the CRATUS trial, which suggested an optimal dose at 100 million cells, with minimal additional benefits at higher dosing (12). This is also in accord with other studies using allogeneic MSCs for other aging-related indications, e.g., cardiomyopathy (51–53). We thus chose a dose-range around the 100M cell dosage in order to understand what might represent a minimal clinically effective dose.
The maximum dosing level is also guided by previous trials, which showed the safety of allogeneic MSCs at the maximum dose evaluated of 200M cells (12). This safety profile is consistent with other clinical results, which demonstrate the general high safety profile of allogeneic MSCs (9, 11).
Study Endpoints
Six-minute Walk Test (6MWT) as Primary Endpoint
The 6MWT is a validated test that is a reliable indicator of frailty status in older adults (54, 55) which is also shown applicable in several other conditions (56–59). The 6MWT is applicable to frailty because the test is an integrated global assessment of cardiac, respiratory, circulatory and muscular capacity. The 6MWT is predominately a test of aerobic capacity, and is a reflection of a patient’s ability to perform activities of daily living (ADLs) (15). In a study evaluating the distance on the 6MWT as a frailty indicator in 60 older adults with heart failure, a positive correlation was found between patients considered to have low endurance (6MWT of <300 m) and a CHS frailty phenotype score of ≥ 3 (p<0.001) (54). There was also a positive correlation for subjects with high endurance (6MWT>300 meters) and non-frailty (p<0.001) (54). Enright et al. (60) reported strong correlations between low body mass and 6MWT, indicating that frail subjects with sarcopenia perform poorly on 6MWT.
Frailty results in impaired mobility leading to dependence on others for ADLs. Typical ADLs that involve walking, such as shopping, require average walking distances of 200–600 meters (61). Such distances included walking to the post office (52 meters), bank (57.1 meters), medical office building (65.8 meters), pharmacy (206 meters), department store (346 meters), and a grocery store (380 meters) (62). National Surveys indicate that at least 40% of community-dwelling adults over 74 have some difficulty walking a quarter mile (400 meters) (63). According to the US Census Bureau, difficulty walking a quarter mile is a standard criteria used to assess disability in the physical domain which is also “the most common lower body functional limitation for adults” (64). Furthermore, slow walking speed is a strong predictor of mortality in older adults (65).
Several studies have determined minimal clinically important differences (MCIDs) in the 6MWT. These range from 17.8 - 20 m in older adults (66, 67), to 32 m for patients with congestive heart failure (68), whereas a decline of 30 m correlates with a 19% increase in mortality for patients with congestive heart failure (69).
Strengths, Limitations, and Risk-Benefits
The trial is powered for efficacy, and endpoints evaluate changes in multiple domains for assessing frailty. It is also rigorously designed to evaluate dose-response relationship of Lomecel-B, centered around a dose that appeared most effective in prior trials (100M cells). This study refines a target patient population based on data from prior trials, which could enhance study powering through reduction in baseline variability.
A general limitation is the lack of interventional trials using an investigational products for frailty, and thus no precedence has been established for registrational endpoints. Subjects in this trial are given a single dose of Lomecel-B or placebo and followed for 9 months. While this dosing frequency and study duration could potentially be limiting to observing an effect, the prior clinical trials support potential efficacy with a single dose over this time-window.
Finally, there are no approved medical therapeutics for frailty in older adults. Allogeneic MSCs have a well-documented high safety profile (11), were shown to be safe and tolerable in successfully completed phase 1 and phase 2 trials for this indication (12, 13) and also showed provisional efficacy in those trials. Given the major and growing unmet medical need of frailty in older adults, and the demonstrated safety and provisional efficacy of this approach, the risk-benefit ratio strongly justifies conducting this next-phase trial.
Summary
This study evaluates several efficacy domains of Lomecel-B for frailty in older adults and represents the first clinical trial powered for efficacy and dose-response establishment of a regenerative medicine product for this growing unmet medical need. The results of this trial will have important implications in an emerging area of geroscience and could lead to novel approaches to enhance healthspan in older individuals thereby reducing the socio-economic societal burden imposed by frailty in the aging population.
References
Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–92.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32.
Dzau VJ, Inouye SK, Rowe JW, Finkelman E, Yamada T. Enabling Healthful Aging for All - The National Academy of Medicine Grand Challenge in Healthy Longevity. N Engl J Med. 2019;381(18):1699–701.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M57.
Hubbard RE, Woodhouse KW. Frailty, inflammation and the elderly. Biogerontology. 2010;11(5):635–41.
Bisset ES, Howlett SE. The biology of frailty in humans and animals: Understanding frailty and promoting translation. Aging Med (Milton). 2019;2(1):27–34.
Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, et al. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine. 2015;2(5):467–75.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90.
Oliva AA, McClain-Moss L, Pena A, Drouillard A, Hare JM. Allogeneic mesenchymal stem cell therapy: A regenerative medicine approach to geroscience. Aging Med (Milton). 2019;2(3):142–6.
Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:100249.
Tompkins BA, DiFede DL, Khan A, Landin AM, Schulman IH, Pujol MV, et al. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Gerontol A Biol Sci Med Sc. 2017;72(11):1513–22.
Golpanian S, DiFede DL, Khan A, Schulman IH, Landin AM, Tompkins BA, et al. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. J Gerontol A Biol Sci Med Sci. 2017;72(11):1505–12.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–95.
American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80(7):837–41.
Cesari M, Landi F, Calvani R, Cherubini A, Di Bari M, Kortebein P, et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin Exp Res. 2017;29(1):81–8.
Shechtman O, Mann WC, Justiss MD, Tomita M. Grip strength in the frail elderly. Am J Phys Med Rehabil. 2004;83(11):819–26.
Lee L, Patel T, Costa A, Bryce E, Hillier LM, Slonim K, et al. Screening for frailty in primary care: accuracy of gait speed and hand-grip strength. Can Fam Physician. 2017;63(1):e51–e7.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94.
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61.
Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429–34.
Batko-Szwaczka A, Dudzińska-Griszek J, Hornik B, Janusz-Jenczeń M, Włodarczyk I, Wnuk B, et al. Frailty phenotype: evidence of both physical and mental health components in community-dwelling early-old adults. Clin Interv Aging. 2020;15:141.
Fragoso CAV, Hsu F-C, Brinkley T, Church T, Liu CK, Manini T, et al. Combined reduced forced expiratory volume in 1 second (FEV1) and peripheral artery disease in sedentary elders with functional limitations. J Am Med Dir Assoc. 2014;15(9):665–70.
Theou O, Cann L, Blodgett J, Wallace LM, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev. 2015 May 1;21:78–94.
Chen S, Honda T, Chen T, Narazaki K, Haeuchi Y, Supartini A, et al. Screening for frailty phenotype with objectively-measured physical activity in a west Japanese suburban community: evidence from the Sasaguri Genkimon Study. BMC Geriatr. 2015;15:36.
Fairhall N, Kurrle SE, Sherrington C, Lord SR, Lockwood K, John B, et al. Effectiveness of a multifactorial intervention on preventing development of frailty in pre-frail older people: study protocol for a randomised controlled trial. BMJ Open. 2015;5(2):e007091.
Castell MV, Sanchez M, Julian R, Queipo R, Martin S, Otero A. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Fam Pract. 2013;14:86.
Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch Intern Med. 1999;159(15):1701–4.
Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15(9):555–65.
Cesari M, Leeuwenburgh C, Lauretani F, Onder G, Bandinelli S, Maraldi C, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83(5):1142–8.
Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3.
Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis. 2015;74(1):104–7.
Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34(6):614–9.
Symonds T, Boolell M, Quirk F. Development of a questionnaire on sexual quality of life in women. J Sex Marital Ther. 2005;31(5):385–97.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–30.
Lee DM, Tajar A, Ravindrarajah R, Pye SR, O’Connor DB, Corona G, et al. Frailty and sexual health in older European men. J Gerontol A Biol Sci Med Sci. 2013;68(7):837–44.
Bretz F, Pinheiro JC, Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics. 2005;61(3):738–48.
Menon SM, Zink RC. Modern Approaches to Clinical Trials Using SAS: Classical, Adaptive, and Bayesian Methods: SAS Institute; 2105.
Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci. 2007;62(7):731–7.
Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–36.
Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26.
Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–62.
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62.
Tay L, Lim WS, Chan M, Ye RJ, Chong MS. The Independent Role of Inflammation in Physical Frailty among Older Adults with Mild Cognitive Impairment and Mild-to-Moderate Alzheimer’s Disease. J Nutr Health Aging. 2016;20(3):288–99.
Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–9.
Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–9.
Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011;27(1):27–37.
Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15.
Kervio G, Carre F, Ville NS. Reliability and intensity of the six-minute walk test in healthy elderly subjects. Med Sci Sports Exerc. 2003;35(1):169–74.
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, T et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. J Am Med Assoc. 2012;308(22):2369–79.
Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127(2):213–23.
Natsumeda M, Florea V, Rieger AC, Tompkins BA, Banerjee MN, Golpanian S, et al. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration. J Am Coll Cardiol. 2017;70(20):2504–15.
Boxer RS, Wang Z, Walsh SJ, Hager D, Kenny AM. The utility of the 6-minute walk test as a measure of frailty in older adults with heart failure. Am J Geriatr Cardiol. 2008;17(1):7–12.
Chan WL, Pin TW. Reliability, validity and minimal detectable change of 2-minute walk test, 6-minute walk test and 10-meter walk test in frail older adults with dementia. Exp Gerontol. 2019;115:9–18.
Dunaway Young S, Montes J, Kramer SS, Marra J, Salazar R, Cruz R, et al. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve. 2016;54(5):836–42.
Agrawal MB, Awad NT. Correlation between six minute walk test and spirometry in chronic pulmonary disease. J Clin Diagn Re. 2015;9(8):OC01.
Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension: comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161(2):487–92.
Kierkegaard M, Tollbäck A. Reliability and feasibility of the six minute walk test in subjects with myotonic dystrophy. Neuromuscul Disord. 2007;17(11–12):943–9.
Enright PL. The Six-Minute Walk Test. Respir Care. 2003;48(8):783–5.
Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314–22.
Bohannon RW, Bubela D, Magasi S, McCreath H, Wang YC, Reuben D, et al. Comparison of walking performance over the first 2 minutes and the full 6 minutes of the Six-Minute Walk Test. BMC Res Notes. 2014;7:269.
Gardener EA, Huppert FA, Guralnik JM, Melzer D. Middle-aged and mobility-limited prevalence of disability and symptom attributions in a national survey. J Gen Intern Med. 2006;21(10):1091–6.
Taylor DM. Americans with disabilities: 2014. US Census Bureau. 2018:1–32.
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. J Am Med Assoc. 2011;305(1):50–8.
Kwok BC, Pua YH, Mamun K, Wong WP. The minimal clinically important difference of six-minute walk in Asian older adults. BMC Geriatr. 2013;13:23.
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–9.
Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J. 2013;24(3):21.
Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16(5):208–13.
Acknowledgments
We thank Dr. Bruce Babbitt and Martin Roessner of PAREXEL for their assistance in the design and statistical approach for this trial.
Funding
Funding: This trial was funded by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) Small Business Innovation Research (SBIR) grant number 4R44AG062015 and Small Business Technology Transfer (STTR) grant number 1R42AG054322.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest: AAO, BH, LM-M, KNR, KY, LD, GAG and JMH are affiliated with Longeveron Inc. JMH is a co-founder, board member and paid consultant of Longeveron Inc. JMH is also inventor of technology licensed to Longeveron Inc. This relationship is reported to the University of Miami, and a management plan is in place. EV and JW are members of Longeveron’s Science Advisory Board, for which they receive personal fees (honoraria).
Ethical standard: Informed consent is obtained from all the participants involved in the study. Ethics approval for this study is provided by the Institutional Review Board (WIRB).
Rights and permissions
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Yousefi, K., Ramdas, K.N., Ruiz, J.G. et al. The Design and Rationale of a Phase 2b, Randomized, Double-Blinded, and Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Lomecel-B in Older Adults with Frailty. J Frailty Aging 11, 214–223 (2022). https://doi.org/10.14283/jfa.2022.2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2022.2